1. Introduction
ibrio cholerae is a natural inhabitant of the aquatic environment and this organism is introduced into human populations through the ingestion of contaminated food or water. In human after colonization to the small intestine through the action of a type IV pilus (TCP) it expresses cholera toxin (CT), which causes the electrolyte imbalance and profuse watery diarrhea. In natural aquatic environment Vibrio cholerae forms biofilm for its long survival in the environment and motility plays an important role in biofilm formation by inducing initial attachment to abiotic surface (Mois et al., Vibrio cholerae is a highly motile organism due to presence of a polar flagellum. Motility and virulence have been inferred to be inversely related to each other (Lospalluto and Finkelstein, 1972;Mekalanos et al., 1983;Taylor et al., 1987;Richardson, 1991). But the exact role of motility in pathogenesis is still remained unclear (Prouty et al., 2001).
The flagellum is a complex structure comprising of multiple structural subunits and expression of flagellar genes is also well regulated involving a four-tiered flagellar transcription hierarchy. Three regulatory genes, flrABC (express flagellar regulatory proteins ABC) are additionally required for the flagellar synthesis. FlrA encoded by class I gene is the master regulator of the flagellar transcriptional hierarchy and ? 54 -dependent transcriptional activator of class II genes, which encode mainly the MS ring and export apparatus components and regulatory factors, including FlrC (Millikan et al., 2003). FlrC is a ? 54 -dependent transcriptional activator that is phosphorylated by FlrB (sensor kinase) component and activates class III flagellar genes which encode the basal body and hook, as well as some of the switch and export apparatus components and the FlaA flagellin. Suggestion is there that the Vibrio cholerae polar flagellar filament is composed of five flagellins, but only one of these, FlaA is essential for motility (Klose and Mekalanos, 1998a).
Repression of FlrA and FlrC increase expression of EPS in response to some environmental signals (Watnic et al., 2001). On the other hand in Vibrio fishery FlrA / FlrC are also found to be involved in the motility (Millikan et al., 2003). In the epidemic subset of Vibrio cholerae including some O1 El Tor and O139 strain absence of flagellar structure triggers the expression of exopolysaccharie which is required for the development of the mature biofilm (Yildiz et al., 1999;Watnic et al., 2001). Beside this flaA (responsible for the synthesis of flagellar structural core protein) mutant strain is nonmotile as well as defective in intestinal colonization (Watnick et al., 2001).
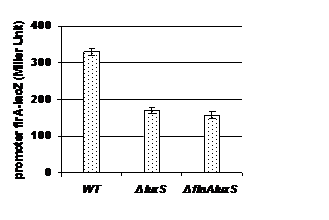
Quorum sensing autoinducer molecules regulate the expression of EPS in Vibrio cholerae O139 Bengal strain MO10. The present study was conducted to investigate whether luxS gene (responsible for the synthesis of quorum sensing autoinducer molecule 2 i.e, AI-2) regulates motility as well as expression of both structural and functional regulatory genes flrA, flrB and flrC. In the peresent study we found that mutation in luxS gene caused reduction in motility and expression of flra was significantly decreased in the luxS mutant strain of the Vibrio cholerae O139 Bengal strain MO10 lac -. However expression of flrB and flrC remained unaltered by luxs gene. So luxS gene may regulate motility through the upregulation of flrA in Vibrio cholerae O139 Bengal strain MO10.
Keywords : Vibrio cholerae O139 Bengal strain MO10 lac-, Î?" luxS, Î?" flaAluxS, motility, Î?" -galactosidase assay.
2. Identification of the Role of LuxS in the Regulation of Motility & the Expression of the Flagellar Structural & Functional Regulators in
3. Vibrio Cholerae
It was previously proposed that cross-species quorum-sensing signaling molecule AI-2 (luxS gene product) stimulates biofilm formation and alters its architecture by stimulating flagellar motion and motility in Escherichia coli (González-Barrios et al., 2006). Moreover luxS gene was also reported to be involved in controlling chemotaxis, flagellar synthesis and motility in E. coli and Helicobactor pylori (Ren et
4. Methods and Materials
a) Bacterial growth MO10 luxS, flaAluxS mutant strain as well as Vibrio cholerae O139 Bengal strain MO10 lac -, a clinical isolate and a strain with high epidemiological importance were used for the present investigation.
Vibrio cholerae O139 Bengal strain MO10 lac -, luxS, flaAluxS were grown in Luria-Bertani (LB) broth, supplemented with streptomycin (100 µg of per ml). All the strains used for this study were displayed in the Vibrio cholerae MO10 lacwild type, Î?"luxS and Î?"flaAluxS strains were transformed with plasmids pSH130, pSH131 and pSH132 respectively, the transcriptional reporter constructs (Table 2). Bacterial cells grown in LB broth were harvested at OD 600 of ~ 0.2 to 0.4, permeabilized with chloroform and sodium dodecyl sulfate and assayed for ?-galactosidase activity following the method mentioned by Miller et al., 1992
5. Result and Discussion
In Vibrio cholerae flagellar synthesis and motility are thought to be important for cholera pathogenesis, but the exact role they play in virulence is still not completely understood. (Lospalluto and Finkelstein, 1972;Mekalanos et al., 1983;Taylor et al., 1987;Richardson et al., 1991;Prouty et al., 2001).
In many bacterial species, flagellar gene transcription occurs in a regulatory hierarchy in which the expression of late genes (i.e., class III or IV) is dependent on the expression of early ones (i.e., class I or II). In Vibrio cholerae, the ? 54 -dependent transcriptional activator FlrA is the sole class I gene which regulates the expression of FlrABC regulon (Prouty et al., 2001;Josenhans et al., 2002;Stewart et al., 1996). FlrA, FlrB and FlrC are reported to be additionally required for the expression of both flagellar structure and function in Vibrio cholerae. The flagellar motor regulates the exopolysaccharide expression. EPS expression was reported to be involved in biofilm formation and this in turn also affects the virulence of the organism because motility was required for its behavior of colonization (Klose & Mekalanos, 1998a). Report shows that repression of either FlrA-or FlrC-dependent transcription ceases flagellar synthesis and produces rugose colony in wild type Vibrio cholerae O139 (Watnic et al., 2001). Mutations in flagellar genes at any level results in the absence of a complete flagellar filament and also produce rugose colony morphology in the Vibrio cholerae O139 strain MO10 (Watnic et al., 2001).
Quorum sensing cell signaling molecule autoinducer 2 (luxS gene product) is also previously reported to control EPS expression, biofilm formation as well as motility in Helicobactor pylori as well as E. Coli (Ren et al., 2004;Osaki et al., 2006;Sperandio et al., 2001, Additionally it was also proposed that luxS gene (responsible for the synthesis of quorum-sensing signal AI-2) is involved in the regulation of EPS expression as well as biofilm formation in Vibrio cholerae O139 Bengal strain MO10 lac -(Biswas et al., 2012 unpublished). So, on the basis of this background we were interested to investigate the role of luxS (responsible for the synthesis of quorum sensing autoinducer molecule 2 i.e, AI-2) in motility as well as in the expression of both flagellar structural and functional regulatory genes i.e flrA, flrB and flrC. In our study we found that motility was reduced in the luxS mutant strain of Vibrio cholerae O139 Bengal strain MO10 lacthan that of the wild type (fig- 1). Beside this we also found that expression of flrA is significantly decreased after deletion in luxS gene in Vibrio cholerae
6. Conclusion
It can be concluded that luxS gene may regulate the motility through the upregulation of trancription of flrA in Vibrio cholerae O139 Bengal strain MO10 lacbut transcription of flrB and flrC were not influenced by luxS gene.

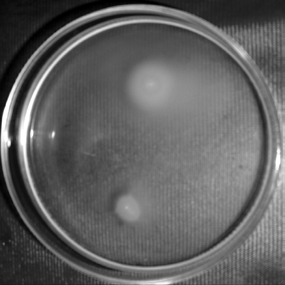
| b) Detection of Motility | |||
| Motility of the Vibrio cholerae strains were | |||
| tested by the method mentioned by Rasid et al. (2003) | |||
| using swarm plate containing 0.3 % LB agar. | |||
| c) Plasmid construction | |||
| The | promoter-lacZ | fusion | containing |
| transcriptional reporter plasmid of flrA, flrB & flrC were | |||
| prepared by PCR amplification of the respective | |||
| promoter using primer pairs Promoter A and B for | |||
| corresponding gene (Table 1). PCR generated fragment | |||
| was digested with EcoRI and BamHI and ligated into the | |||
| corresponding sites of plasmid vector pRS551 (Simons | |||
| performed using MO10 chromosomal DNA as template | |||
| Target | Primer sequence ( 5'-3') |
| gene or | |
| encoding | |
| region | |
| FlrA | GC GAATTCGCTCTAGATAGTTTCGCTAA |
| Promoter-A | |
| FlrA | GCGGATCCGCTCTAGACAAGCGAACCAT |
| Promoter-B | |
| FlrB | GCGAATTCGCAGATCTTGGGTTGGCTTC |
| Promoter-A | |
| FlrB | GCGGATCCGCAGATCTCAGCCAGAGCCT |
| Promoter-B | |
| FlrC | GC GAATTC GCCACTCATAACCCGCTAAA |
| Promoter-A | |
| FlrC | GCGGATCC CCAGAGTCGTTGCAGCACAA |
| Promoter-B |
| Strains | Description | Source |
| Vibrio cholerae | ||
| strains |