1. Introduction
he liver is an organ of paramount importance not only for its metabolism of various xenobiotics and environmental pollutants [1] but for its unique and considerable regenerative capacity, even a moderate cell injury is not reflected by measurable change in its metabolic functions. However, some of its functions are so sensitive that abnormalities start appearing depending upon the nature and degree of its initial damage [2] .
Reactive Oxygen Species (such as H 2 O 2 , O 2 2-, and OH -, collectively known as ROS) play important physiological functions and can also cause extensive cellular damage. Cells are provided with efficient molecular strategies to control strictly the intra cellular ROS level and to maintain the balance between oxidant and antioxidant molecules. Oxidative stress, resulting from an imbalance between the generation of ROS and the antioxidant defense capacity of the cell [3] , effects major cellular components including lipids, proteins and DNA. This phenomenon is closely associated with a number of human disorders such as many degenerative diseases including cardiovascular disease, diabetes, cancer, neurodegenerative disorders [4,5] and with almost all liver pathologies [6][7][8] . All these conditions appear mostly related to chronic oxidative stress. However, the acute exposure to high levels of ROS seems to be responsible for the development of different damages such as during ischemis/reperfusion is acute hepatotoxic agent, which induces peroxidative degeneration of membrane lipids causing hypo perfusion of the membrane. Cytosolic enzyme like SGPT, SGOT and ALP.
A number of medicinal plants are used in traditional system of medicinal for the management of liver disorders. Nature has given us a large number of medicinal plants, some of which are yet to be explored and validated for their medicinal value. The 21 st century has seen a paradigm shift toward therapeutic evaluation of herbal products in liver diseases, carefully synergizing the strengths of traditional medicine with the modern concept of evidence based medical evaluation, standardization and randomized placebo controlled clinical trials to support clinical efficacy. Several herbs are known to possess antioxidant properties and may be useful as liver protective agents [10] .
The herbs containing antioxidant principles are reported to be highly effective in preventing or curing the liver toxicities due to above mentioned challenges. In the present study, the herb Phyllanthus amarus containing polyphenolic compounds is selected to assess hepatoprotective activity [11] .
2. II.
3. Materials and Methods
4. a) Plant Material
The seeds of Phyllanthus amarus were collected from local gardens of Tirupathi. The plant was b) Preparation of Extracts The crushed and dried seeds of Phyllanthus amarus were divided into two parts; one part was extracted successively with petroleum ether, benzene, chloroform and finally with methanol by soxhlet extraction and concentrated by rotary vacuum [12] . The other part is extracted by cold maceration process for aqueous extraction [13] . The obtained extracts were dried by evaporation. The yield 30% w/w and 15.6% w/w were stored in refrigerator and weighed quantities were suspended in tween 80 and 2% tragacanth solution respectively for the experiment. The extracts were used for In-vitro and In-vivo studies to analyze the reparative activity of liver injury due to CCl 4 treated rats.
5. c) Experimental Animals
Male albino rats weighing 130-160g were obtained from the animal house of Nizam Institute of Pharmacy, Hyderabad and housed in Polycarbonate cages. The rats had free access to standard pellet chow and water ad libitum throughout the experiment with the exception of some experiments (see below) in which the animals were deprived of food, but not water, for 18-24 hr. before the experiments were performed. After procurement, all the animals were divided into different groups and were left for one week for acclimatization to experimentation room and were maintained on standard conditions (23 o c, 60%-70% relatively humidity and 12 hr. photo period). There were five animals in each group for observational screening. All experimental protocols described below were approved by the ethical board.
6. d) Acute Oral Toxicity Studies
The acute oral toxicity study is determined according to the guidelines of Organization for Economic Co-operation & Development (OECD) following the up & down method (OECD guideline No. 423). Based on the method, a limit test was performed to categorize the toxicity class of the compound and then main test was performed on three female rats to estimate the exact LD50. The animals were fasted overnight with free access to water, weighed and a single dose of the test substance was administered. Animals were observed individually during first 30 min, periodically during 48 h with special attention given during first 4 h (short-term toxicity) and daily, thereafter for total of 14 days (short-term toxicity). LD50 was found to be greater than 2500 mg/kg, in limit test. The test substance could be classified in the hazard classification as Class 5 -2000 mg/kg <LD50 <5000 mg/kg in the globally harmonized system (GSH). LD50 of test drug was found to be 2500mg/kg from main test [14] .
7. e) Hepatotoxins
It is emphasized that hepatotoxin that cause acute hepatitis should have close resemblance with the viral hepatitis, clinically, biochemically and histopathologically. Certain drugs are also responsible for many hepatic diseases, such as chronic hepatitis, fatty liver, cirrhosis and certain vascular lesions of liver. In many instances drug induced hepatitis is distinguishable from viral hepatitis chemical injured viral hepatitis for experimental studies should be severe enough to cause cell death or to modify hepatic functions. The mechanism of acute hepatic injury depends upon the chemical compound and the species of animal used. We have studied the hepatoprotective activity against CCl 4 induced hepatotoxicity.
8. III. Methods for the Hepatoprotective Evaluation ear 2012 Y
CCl 4 is one of the most powerful hepatotoxin in terms of severity of injury it causes toxic necrosis leading to biochemical changes having clinical features similar to those of acute viral hepatitis [ ] , liver injury was produced by administration of CCl 4 mixed with tween 80. Animals were given single doses of CCl 4 2 mg/kg, i.p per day throughout the experimental setup. Control animals received an equal volume of tween 80.
9. a) In-vitro antioxidant activity
In-vitro models were carried out to evaluate antioxidant activity are Reducing power, Superoxide anion scavenging activity, Hydroxyl radical scavenging activity and Nitric oxide radical scavenging activity.
10. Reducing power
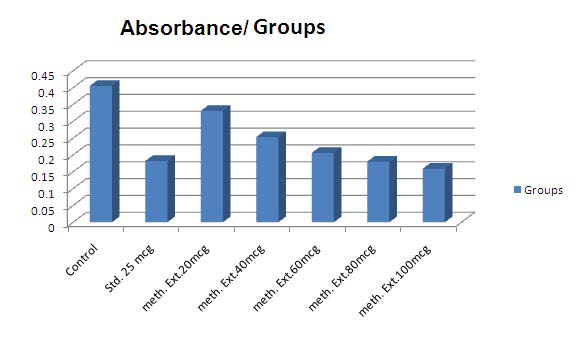
The reducing power of methanolic and aqueous extracts of Phyllanthus amarus seeds were determined according to the method of Oyaizu [ ] Procedure: Extracts of Phyllanthus amarus seeds were mixed in 1ml of distilled water so as to get 20µg, 40µg, 60µg, 80µg, and 100µg concentration. This was mixed with phosphate buffer (2.5ml, 0.2M, pH 6.6) and potassium ferricyanide (2.5ml, 1%). The mixture was incubated at 50ºC for 20 minutes. A portion (2.5ml) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm for 10 minutes.
The upper layer of the solution (2.5ml) was mixed with distilled water (2.5ml) and FeCl 3 (0.5ml, 0.1%), and the absorbance (OD) was measured at 700nm. Increased absorbance of the reaction mixture indicates increased in reducing power.
The percent reducing power was calculated by using the formula:
% increase in absorbance = Control OD-Test OD Control OD × 10011. Superoxide anion scavenging activity
Oxygen is essential for the survival of aerobic cells, but it has long been known to be toxic to them 16 1.
12. 2.
when supplied at concentration greater than those in normal air. The biochemical mechanisms responsible for oxygen toxicity include lipid peroxidation and the generation of H 2 O 2 + the superoxide radical, O 2 + . This superoxide radical can inhibit or propagate the process of lipid peroxidation. Measurement of superoxide anion scavenging activity of Phyllanthus amarus seeds was done by using the method explained by Nishimiki [ ] and modified by Ilhami et al., Procedure: About 1ml of nitroblue tetrazolium (NBT) solution (156µM NBT in 100mM phosphate buffer, pH 7.4), and 0.1ml of sample solution of methanolic extract of Phyllanthus amarus seeds and standard in water was mixed. The reaction was started by adding 100µl of phenazine methosulphate (PMS) solution (60µM PMS in 100mM phosphate buffer, pH7.4) to the mixture. The reaction mixture was incubated at 25ºC for 5 minutes, and the absorbance at 560nm was measured against blank.
Decreased absorbance of the reaction mixture indicated increased superoxide anion scavenging activity. % inhibition of OD was calculated by using the formula mentioned earlier.
associated with several diseases oxygen reacts with the excess nitric oxide to generate nitrite and peroxynitrite anions, which acts as free radicals. This forms the basis of this experiment.
Procedure: Nitric oxide (NO) radial were generated from sodium nitroprusside solution of physiological pH [20] . Sodium nitroprusside (1ml of 10mM) were mixed with 1ml of methanolic extract of Phyllanthus amarus seeds of different concentration (20-100µg/ml) in phosphate buffer (pH 7.4). The mixture of incubated at 25ºC for 150 min. To 1ml of incubated solution, 1ml Griess?s reagent (1% sulphanilamide, 2% o-phosphoric acid and 0.1% naphthyl ethylene diamine dihydrochloride) was added. Absorbance was read at 546nm. % inhibition of OD was calculated by using the formula mentioned earlier.
13. IV. Results
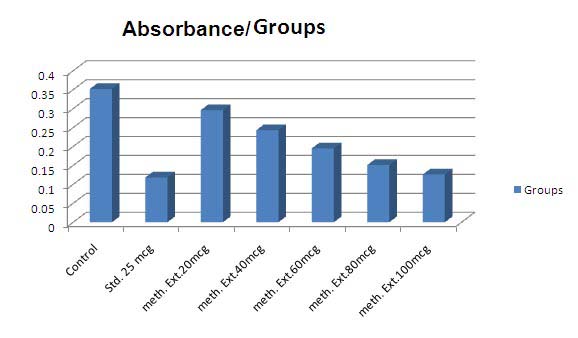
In vitro: It was observed that methanolic extract demonstrated dose dependent increase in the reducing property. 25mcg sodium metabisulphate (Std.) showed 73.09% reducing property. Methanolic extract at 100mcg had more reducing property than 25mcg ear 2012 Y
14. Hydroxyl radical scavenging activity
In biochemical systems, superoxide radical and H 2 O 2 react together to form the hydroxyl radical which can attack and destroy almost all known biochemical [18] Phenylhydrazine when added to erythrocyte hosts cause peroxidation of endogenous lipids and alteration of membrane fluidity. This peroxidation damage to erythrocytes is probably initiated by active oxygen species like O 2
? , OH ? and H 2 O 2 which are generated in solution from auto-oxidation of phenyl hydrazine. This forms the basis of this experiment.
Procedure: Hydroxyl radical generation by phenyl hydrazine has been measured by the 2deoxyribose degradation, assay of Halliwell and Gutteridge [ ] In 50mM phosphate buffer (pH 7.4), 1mM deoxyribose and 0.4ml of methanolic extract of Phyllanthus amarus seeds and standard were taken. 0.2ml phosphate buffer was added to make reaction solution 1.6ml. after 10 min incubation 0.4ml of o.2mM phenyl hydrazine was added. Incubation was terminated after 1hr and 4 hrs and 1ml each of 2.8% TCA and 1% (w/v) thiobarbituric acid were added to the reaction mixture and heated for 10 minutes in a boiling water bath. The tubes were cooled and absorbance was taken at 532nm. Decreased in absorbance is indicating the hydroxyl free radical scavenging activity. The % reduction in the OD is calculated.
15. Nitric oxide radical scavenging activity
Nitric oxide (NO) is an important chemical mediator generated by endothelial cells, macrophages, neurons etc. involved in the regulation of various physiological processes. Excess concentration of NO is
16. b) In-vivo antioxidant activity
The Wister rats were divided into 5 groups of 6 individuals each [21] for 5days study. Table : 1
17. Group
Group I received Tween 80 1ml/kg I.P., on 2 nd and 3 rd day. Group II, III, IV and V received CCl 4 2mg/kg I.P., on 2 nd , 3 rd , days. The Group III, IV, V, received Liv 52 100mg/kg p.o., Methanolic Extract 250mg/kg p.o., Aqueous Extract 250mg/kg p.o., before 30min of toxicant respectively. Animals were sacrificed on the 5 th day under mild ether anesthesia.
The blood samples were collected from retro orbital plexus for evaluating the serum biochemical parameters and liver was dissected out, blotted off blood, washed with saline and stored in 10% formalin and preceded for histopathology to evaluate the details of hepatic architecture in each group microscopically.
18. c) Statistical analysis
The statistical analysis was carried out by oneway analysis of variance (ANOVA). The values are represented as mean ± SE. Comparison of mean values of different groups treated with different dose levels of extracts and positive controls were estimated by Tukey's Multiple Comparison Test. P < 0.05 was considered significant. sodium metabisulphate i.e. 88.88%.
There was percentage increase in the superoxide anion scavenging activity. Methanolic extract showed lesser activity than standard i.e. 70.64%.
It was observed that methanolic extract demonstrated dose dependant percentage increase in the hydroxyl radical scavenging activity in case of 1 hr. incubation period i.e. 64.20% But, showed higher in case of 4 hrs incubation period i.e. 55. 55% It was observed that that methanolic extract demonstrated dose dependant percentage increase in the nitric acid radical scavenging activity. 25mcg sodium metabisulphate (Std.) showed 70.52% activity. Methanolic extract had more nitric oxide radical scavenging activity at 100 mcg than compared to 25 mcg sodium metabisulphate. i.e. 71.78%.
In vivo: There was increased level of SGPT in CCl4 treated group 312.420U/L. The SGPT level was restored to 77.84 U/L by 250mg/kg methanolic extract of the seeds which was near to effect of 100mg/kg Liv.52 i.e. 65.395 U/L.
SGOT levels increased significantly in CCl4 treated group i.e. 318.412 U/L. methanolic extract of the seeds reduced the elevated level of SGOT to 72.69 U/L, which was very near to100mg/kg Liv.52 i.e. 71.212 U/L.
In case of bilirubin, methanolic extract reduced the level of bilirubin by 4.892mg/dl to 1.20mg/dl.
There was increase in ALP level observed in CCl4 treated group (235.86 IU/L). ALP level was restored to 97.44 IU/L by methanolic extract of the seeds which was near to effect of 100mg/kg Liv.52 i.e.
19. IU/L.
There was no significant rise in total cholesterol and triglycerides levels in CCl4 treated group. Significant effect was observed with in methanolic extract and aqueous extract was comparable with 100mg/kg Liv.52.
Liver section of control rat showed normal hepatocytes and normal architecture (Figure 1A). Liver sections from CCl4 treated rats demonstrated the destruction of architectural pattern, nodule formation in the lobular zone, inflamed periportal zone, and moderate inflammation of portal area (Figure 1B). Liver sections from Liv 52 treated rats showed regeneration of normal hepatocytes (Figure 1C). Liver sections from a methanolic extract of Phyllanthus amarus seeds treated rat showing normal lobular architecture (Figure 1D). Liver section from an aqueous extract of Phyllanthus amarus seeds treated rat showing normal lobular architecture no necrosis or fatty changes or any inflammatory reaction can be seen. (Figure 1E). These histopathological findings demonstrate a hepatoprotective effect of the extracts against CCl4mediated liver damage.
20. VI.
21. Discussion
The purpose of this study was to explore the hepatoprotective effect of extracts of Phyllanthus amarus seeds in the hepatic damage caused by CCl 4 .
Administration of CCl 4 to normal rats increased serum levels of AST, ALT, ALP, and bilirubin. The enzymes leaking out from damaged liver cells into circulating blood represent the damage to hepatic cells. It is well established that the toxic metabolite of CCl 4 , a free radical CCl 3 is responsible for damage to liver cells. Invitro models like Reducing power, Superoxide anion scavenging activity, Hydroxyl radical scavenging activity and Nitric oxide radical scavenging activity were carried out with methanolic extract of Phyllanthus amarus seeds for its antioxidant properties. A protective activity could be demonstrated in the CCl 4 induced liver damage in rats. In-vivo methanolic and aqueous extracts of the seeds of Phyllanthus amarus 250mg/kg were found to have protective properties in rats with CCl 4 induced liver damage and caused statistically significant decrease in all the above parameters.
Liver section of control rat showed normal hepatocytes and normal architecture (Figure 1A). Liver sections from CCl4 treated rats demonstrated the destruction of architectural pattern, nodule formation in the lobular zone, inflamed periportal zone, and moderate inflammation of portal area (Figure 1B). Liver sections from Liv 52 treated rats showed regeneration of normal hepatocytes (Figure 1C). Liver sections from a methanolic extract of Phyllanthus amarus seeds treated rat showing normal lobular architecture (Figure 1D).
Liver section from an aqueous extract of Phyllanthus amarus seeds treated rat showing normal lobular architecture no necrosis or fatty changes or any inflammatory reaction can be seen. (Figure 1E). These histopathological findings demonstrate a hepatoprotective effect of the extracts against CCl4mediated liver damage. The methanolic and aqueous extracts of Phyllanthus amarus seeds do have a protective capacity both In-vitro and In-vivo models of CCl 4 mediated liver injury VII. Histopathological Studies in ccl4 Induced Hepatotoxicity

| Group | Absorbance | % |
| Mean±SEM | Increase | |
| Control | 0.171±0.001 | --- |
| Control + Std 25µg | 0.296±0.002*** | 73.09 |
| Control + methanolic extract 20µg | 0.182±0.001* | 6.43 |
| Control + methanolic extract 40µg | 0.216±0.002*** | 26.31 |
| Control + methanolic extract 60µg | 0.259±0.002*** | 51.46 |
| Control + methanolic extract 80µg | 0.283±0.002*** | 65.49 |
| Control + methanolic extract 100µg | 0.323±0.002*** | 88.88 |
| Values are the mean ± S.E.M., n=3 | ||
| Significance***P<0.001 and * P<0.05 compared to control. Std: Sodium metabisulphate | ||
| Group | Absorbance Mean±SEM | % Increase |
| Control | 0.862±0.020 | --- |
| Control + Std 25µg | 0.225±0.001*** | 73.89 |
| Control + methanolic extract 20µg | 0.469±0.001*** | 45.59 |
| Control + methanolic extract 40µg | 0.422±0.002*** | 51.04 |
| Control + methanolic extract 60µg | 0.377±0.002*** | 56.26 |
| Control + methanolic extract 80µg | 0.319±0.002*** | 62.99 |
| Control + methanolic extract 100µg | 0.253±0.002*** | 70.64 |
| Values are the mean ± S.E.M., n=3 | ||
| Significance***P<0.001 compared to control. Std: Sodium metabisulphate | ||
| Group | Absorbance | % | Absorbance | % | |
| Mean±SEM | Inhibition | Mean±SEM | Inhibition | ||
| Control | 0.352±0.0005 | --- | 0.405±0.0020 | --- | |
| Control + Std 25µg | 0.118±0.0037*** | 66.47 | 0.182±0.0040*** | 55.06 | |
| Control + methanolic extract | 0.296±0.0028*** | 15.90 | 0.331±0.003*** | 18.27 | |
| 20µg | |||||
| Control + methanolic | extract | 0.243±0.0020*** | 30.96 | 0.253±0.001*** | 28.14 |
| 40µg | |||||
| Control + methanolic extract | 0.195±0.0030*** | 46.59 | 0.206±0.002*** | 37.53 | |
| 60µg | |||||
| Control + methanolic extract | 0.151±0.002*** | 57.10 | 0.180±0.003*** | 49.13 | |
| 80µg | |||||
| Control + methanolic extact | 0.126±0.0026*** | 64.20 | 0.159±0.0041*** | 55.55 | |
| 100µg | |||||
| Values are the mean ± S.E.M., n=3 | |||||
| Significance***P<0.001 compared to control. Std: Sodium metabisulphate | |||||
| Group | Absorbance | % |
| Mean±SEM | Increase | |
| Control | 0.397±0.0015 | --- |
| Control + Std 25µg | 0.117±0.0015*** | 70.52 |
| Control + methanolic extract 20µg | 0.242±0.0008*** | 39.04 |
| Control + methanolic extract 40µg | 0.218±0.0017*** | 45.08 |
| Control + methanolic extract 60µg | 0.186±0.0017*** | 53.14 |
| Control + methanolic extract 80µg | 0.142±0.0026*** | 64.23 |
| Control + methanolic extract 100µg | 0.112±0.0028*** | 71.78 |
| Figure : 5 |
| induced hepatotoxicity | |||||||
| Biochemical parameters Mean±SEM | |||||||
| Treatment | SGPT | SGOT | ALP | Bilirubin | Protein | Cholesterol | Triglycerides |
| U/L | U/L | IU/L | (mg/dl) | (g/dl) | (mg/dl) | (mg/dl) | |
| Negative control | 55.558 | 55.216 | 122.29 | 0.926 | 8.88 | 110.88 | 171.22 |
| 1ml/kg Tween 80 | ± | ± | ± | ± | ± | ± | ± |
| 3.331 | 5.617 | 6.486 | 0.029 | 0.34 | 10.771 | 7.198 | |
| Positive control | 312.42 | 318.412 | 235.86 | 4.892 | 5.85 | 172.62 | 190.36 |
| CCl4 treated 2ml/kg | ± | ± | ± | ± | ± | ± | ± |
| 14.275 | 13.543 | 8.207 | 0.451 | 0.40 | 10.522 | 7.516 | |
| CCl4 + Liv. 52 | 65.395 | 71.212 | 95.68 | 1.546 | 8.46 | 118.25 | 145.48 |
| 2ml/kg + 100mg/kg | ± | ± | ± | ± | ± | ± | ± |