1. Introduction
he liver is an organ of paramount importance. Due to its unique and considerable regenerative capacity, even a moderate cell injury is not reflected by measurable change in its metabolic functions. However, some of its functions are so sensitive that abnormalities start appearing depending upon the nature and the degree of initial damage. The factors as nutritional, biochemical, bacteriological, viral, or environmental aberration. The liver plays a significant role not only in the metabolism and disposition of the chemicals to which it is exposed directly or indirectly, but also in the metabolism of fats, carbohydrates, proteins, and immune-modulation. The impairment of the liver function is generally caused by xenobiotics, excessive exposure to various pharmacological and chemical agents, and protozoal or viral infections. Depending upon the severity of cellular injury, acute hepatitis can lead to chronic hepatitis, which is finally terminated to cirrhosis or malignant lesions in untreated cases. Alcoholic liver disease (ALD) is one of the most serious consequences of chronic alcohol abuse. Liver cirrhosis, the culmination of the illness, is one of the leading causes of death in western countries. 1,2 Hepatic fibrosis occurs in the advanced liver disease, where normal hepatic tissue is replaced with collagen rich extracellular matrix (ECM) and, if left untreated, result in cirrohsis. 3,4 Cirrhosis is a complication of many liver diseases that is characterized by abnormal structure and function of the liver. The diseases that lead to cirrhosis do so because they injure and kill liver cells and the inflammation and repair that is associated with the dying liver cells causes scar tissue to form. The liver cells that do not die multiply in an attempt to replace the cells that have died. This results in clusters of newly-formed liver cells (regenerative nodules) within the scar tissue. There are many causes of cirrhosis; they include chemicals (such as alcohol, fat, and certain medications), viruses, toxic metals (such as iron and copper that accumulate in the liver as a result of genetic diseases), and autoimmune liver disease in which the body's immune system attacks the liver. The magnitude of derangement of liver by disease or hepatotoxins are generally measured by the level of glutamate pyruvate transaminase (ALT), glutamate oxaloacetate transaminase (AST), alkaline phosphatase (ALP), bilirubin, albumin, and whole liver homogenate.
Medicines that are used today are not definitely the same as those that were used in ancient times or even in the recent past. India has a wealth of medicinal plants most of which have been traditionally used in Ayurveda, Unani systems of medicine and by tribal mentioned that every plant on this earth is useful for human beings, animals and other plants. The liver is the key organ regulating homeostasis in the body. It is involved with almost all the biochemical pathways related to growth, fight against diseases, nutrient supply, energy provision and reproduction 5 . The liver is expected not only to perform physiological functions but also to protect the hazards of harmful drugs and chemicals. In spite of tremendous scientific advancement in the field of hematology in recent years, liver problems are on the rise. Jaundice and hepatitis are two major hepatic disorders that account for a high death rate. 6 Herbal drugs are playing an important role in health care programs worldwide, and there is a resurgence of interest in herbal medicines for treatment of various ailments including hepatopathy. India, the abode of Ayurvedic system of medicine, assigns much importance to the pharmacological aspects of many plants. Hepatoprotective effect of some plants like Spirulina maxima 7 , Eclipta alba 8 , Boehmeria nivea 9 , Cichorium intybus 10 , and Picrorhiza kurroa 11 has been well established. Nearly 150 phytoconstituents from 101 plants have been claimed to possess liver protecting activity 12 . At the same time, surprisingly, we do not have satisfactory plant drugs/formulations to treat severe liver diseases. Most of the studies on hepatoprotective plants are carried out using chemical induced liver damage in rodents as models. A few excellent reviews have appeared on this subject in the recent past 13 . This study is based on the natural products responsible for repairing and healing of adversely affected liver cells. In the present study, we selected two plants namely S. mukorossi and R. emodi and investigated the hepatoprotective effect of these plant extracts against CCl4 induced hepatocyte damage in vitro and liver injury in vivo.
2. S. mukorossi
Gaerten (Sapindaceae), commonly known as Ritha or Aritha is found throughout India. The major constituents of its fruit are saponins (10%-11.5%), sugars (10%) and mucilage 14 . The fruit of the plant is reported to have expectorant, emetic, alexipharmic, and abortificiant effects. It is also used in excessive salivation, epilepsy and chlorosis 15 , 16 .
Saponins from this plant are known to be spermicidal in vitro 17 . This spermicidal property has been used in contraceptive cream 18 . The alcoholic extract (Sapindus trifoliatus Linn) is reported to possess antiimplantation activity.
3. R. emodi (Polygonaceae) commonly known as
Indian or Himalayan Rhubarb is found in India. The major constituents of rhubarb rhizomes are anthraquinones. Rhubarb is used as a laxative, diuretic to treat kidney stones, gout, and liver diseases characterized by jaundice. Externally, it is used to heal skin sores and scabs. Paradoxically, although larger treat dysenteric diarrhea 19 . Chinese use rhubarb as an ulcer remedy and consider it a bitter, cold, dry herb used to "clear heat" from the liver, stomach and blood, to expel helminthes and to treat cancer, fever, upper intestinal bleeding (ulcers), and headache 20 , 21 . It is also used to treat toothache 22 . In Europe, rhubarb is a component of spring tonics or blood cleansing cures, including Swedish bitter 23 . Turkish or medicinal rhubarb is also one of the four major ingredients in the herbal cancer remedy. We isolated the extracts from both plants, and a study was designed using the extracts of S. mukorossi and R. emodi to assess the curative effect of Sapindus mukorossi and Rheum emodi extracts in CCl4 induced liver cirrhosis in male rats.
4. II.
Materials and Methods a) Plant materials Authentic samples of S. mukorossi and R. emodi were obtained from authorized supplier M/s Munnalal Dawasas and Co. Hyderabad, Andhra Pradesh, India. The plants were previously identified and authenticated by experts in the Post Graduate and Research Department of Botany, Anwarul-loom College Hyderabad, Andhra Pradesh, India.
5. b) Animals
Male Wister rats weighing 175-200 g were obtained from the animal house of Deccan College of Medical Sciences, Hyderabad and housed in polycarbonate cages. The rats had free access to standard pellet chow and water ad libitum throughout the experiment with the exception of some experiments (see below) in which the animals were deprived of food, but not water, for 18-24 h before the experiments were performed. After procurement, all the animals were divided into different groups and were left for one week for acclimatization to experimentation room and were maintained on standard conditions (230, 60%-70% relative humidity and 12 h photo period). There were six animals in each group for observational screening and acute toxicity studies. All experimental protocols described below were approved by the ethical board.
6. c) Extraction, separation, and purification of the compounds
For phytochemical analysis, approximately 100 g of fruit pericarp of S. mukorossi and rhizomes of R. emodi was collected and materials were chopped, air dried at 35-40 0 and pulverized in electric grinder. The powder obtained was successively extracted with the following chemicals, petroleum ether (60-80) 0 , benzene, chloroform, and ethanol, respectively. The extracts were then powdered by using rotary evaporator under reduced pressure. respectively. All the filtrates obtained were dried by evaporation (Rotometer, 40 0 ), the dried extracts were individually dissolved in 10 mL ethanol (95%) and then subjected to complete drying process and weighed according to the AOAC (1990) method 20 .
7. d) Hepatotoxins
It is emphasized that hepatotoxins that cause acute hepatitis should have close resemblance with the viral hepatitis, clinically, biochemically, and histologically. Certain drugs are also responsible for chronic hepatic disease such as chronic hepatitis, fatty liver, cirrhosis, and several vascular lesions of the liver. In many instances drug induced hepatitis is indistinguishable from viral hepatitis. Chemically induced hepatic injury for experimental studies should be severe enough to cause cell death or to modify hepatic functions. The mechanism of acute hepatic injury depends upon the chemical compound and the species of animals used. We have studied hepatoprotective activity against carbon tetrachloride (CCl4) induced hepatotoxicity. CCl4 is one of the most powerful hepatotoxin in terms of severity of injury. It causes toxic necrosis leading to biochemical changes having clinical features similar to those of acute viral hepatitis 24 , 25 .
Induction of Liver Cirrhosis in Rats : Cirrhosis was induced by administering CCl4 intragastrically. The initial dose of CCl4 was 40?L/rat, and subsequent doses were adjusted based on the change in body weight as described. 26 Estimation of Hydroxyproline : Hepatic hydroxyproline content was measured as described 27 (Table 1).
Detailed evaluation of Curative effect of Sapindus mukorossi and Rheum emodi in CCl4 five groups of six animals each. Group 1 served as vehicle control and was administered with normal saline. Group 2 rats were given CCl4 40 ?L/rat checking the biochemical parameters periodically for hepatotoxicity.
Group 3 rats were given CCl4 + extracts of S. mukorossi 2.5 g/kg, p.o. Group 4 rats were given CCl4 + extracts of R. emodi 3.0 g/kg, p.o. Blood was collected from the orbital sinus in all animals and serum separated for different estimations (Table 1). The rats were anesthetized and sacrificed after the experimental period by cervical decapitation. The liver tissue was examined histopathologically.
8. e) Statistical analysis
The data obtained was subjected to statistical analysis using ANOVA for comparing different groups (Armitage, 1987) and Dunnett's t test for control and test groups (Dunnett, 1964). The two tailed unpaired student t test for comparing means before and after treatment and one tailed unpaired student t test for comparing control and drug treated group, ED50 value with 95% confidence limits (CL) by regression analysis using log dose response (Swinscow, 1980 & Ghosh, 1984) were used. P < 0.05 or less was taken as the criterion of significance.
9. III. RESULTS
S. mukorossi and R. emodi extracts showed significant hepatoprotective activity against CCl4 induced liver injury in primary hepatocytes cultures 28 . The hepatotoxic effects of CCl4 are attributed to its metabolism by P450 to yield toxic trichloromethyl radicals that can act as free radical initiators 29 . These radicals are believed to induce injury either by interacting with the unsaturated fatty acids of cell membranes, thereby causing lipid peroxidation, or by binding covalently to important macromolecules such as proteins, lipids, or DNA 30 , 31 . The extracts of S. mukorossi and R. emodi reduced the levels of LDH and GPT released from CCl4 injured rat hepatocytes into the medium in a concentration dependent manner, thus signifying their hepatoprotective activity 28 . In CCl4 induced cirrhosis rats, serum activities of AST, ALT, ALP, and Bilirubin were increased significantly when compared to the control (Table 1).
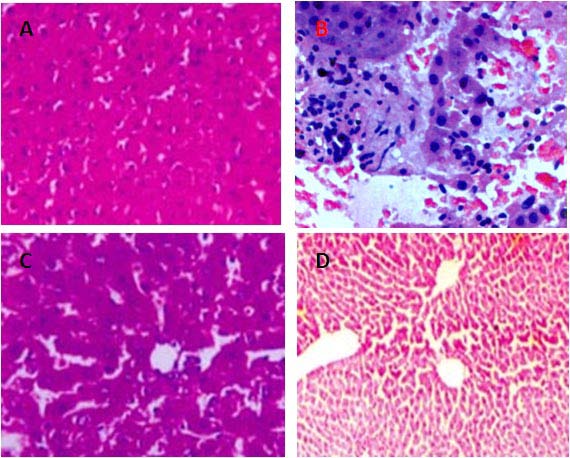
The CCl4 treated group showed a marked increase in serum Bilirubin (mg %) (1.82 ±0.08), ALT (IU/L) (1262.30 ± 1.97), AST (IU/L) (903.50 ± 30.00), and ALP (IU/L) (104.09 ± 3.00) activity indicating the injury caused by CCl4. Treatment with the extracts of S. mukorossi and R. emodi significantly decreased the above elevated parameters and the normal architectural liver pattern was restored as given below. Slide of a control rat showing normal hepatocytes and architecture (Figure 1A). Slide of CCl4 treated rat demonstrating the loss of hepatic architecture with formation of nodules of 1B).
10. Slide of S. mukorossi treated rat showing normal lobular
architecture no necrosis or fatty changes (Figure 1C).
Slide of R. emodi treated rat showing normal lobular architecture.
(Figure 1D). These histopathological findings demonstrate a Curative effect of Sapindus mukorossi and Rheum emodi in CCl4 induced liver cirrhosis.
11. IV. DISCUSSION
The purpose of this study was to explore the Curative effect of Sapindus mukorossi and Rheum emodi in CCl4 induced liver cirrhosis. Administration of CCl4 to normal rats increased serum levels of AST, ALT, ALP, and Bilirubin. The enzymes leaking out from damaged liver cells into circulating blood represent the damage to hepatic cells. It is well established that the toxic metabolite of CCl4, a free radical CCl3 is responsible for damage to liver cells. S. mukorossi and R. emodi extracts caused statistically significant decrease in all the above parameters at the dose of 2.5 Histopathological examination of the liver sections of rats treated with CCl4 Treatment with the extracts of S. mukorossi and R. emodi significantly decreased the above serum elevated parameters and the normal architectural liver pattern was restored. Slide of a control rat showing normal hepatocytes and architecture (Figure 1A). Slide of CCl4 treated rat demonstrating the loss of hepatic architecture with formation of nodules of hepatocytes without lobular pattern and no central veins, necrosis, thin fibrous bands encircling nodules of hepatocytes, micro-nodular cirrhosis of liver (Figure 1B). Slide of S. mukorossi treated rat showing normal lobular architecture no necrosis or fatty changes (Figure 1C). Slide of R. emodi treated rat showing normal lobular architecture.
(Figure 1D). Hepatic hydroxyproline content of the normal rats and the CCl4 treated were compared with the treated extracts of Sapindus mukorossi and Rheum emodi it is an evident that the extracts of Sapindus mukorossi and Rheum emodi are having the curative effect on CCl4 induced liver cirrhosis. Further it should be evaluate in the human studies in order to have the proper treatment for the liver diseases. mg/kg and 3.0 mg/kg given orally to CCl4 treated rats.

| of the fruit pericarp of S. mukorossi (SM) and rhizomes | |
| of R. emodi (RE) were column chromatographed over | |
| Silica gel (200 mesh), eluting with CHCl3-MeOH (70:30, | |
| 60:40, 50:50, 25:75) and compound fractions of (250 | |
| mL each) were collected and monitored by TLC. These | |
| column chromatographed compound fractions were | |
| further filtered to yield saponins from S. mukorossi and | |
| anthraquinones from R. emodi, which were separated | |
| by paper chromatography and preparative TLC to yield | |
| saponins [(SM-A (petroleum ether), SM-B (benzene), | |
| SM-C (chloroform) & SM-D (ethanol)], and | |
| anthraquinones | [(RE-A (petroleum ether), RE-B |
| (benzene), RE-C (chloroform) & RE-D (ethanol)], | |
| ear 2012 |
| Y |
| Medical Research Volume XII Issue VIII Version I |
| Global Journal of |
| Treatment | Dose(mg/kg, | Serum Parameters | Liver weight | |||
| p.o .) | ||||||
| Hepatic | ||||||
| ALT (IU/l) | AST (IU/l) | ALP (IU/l) Bilirubin (mg) | hydroxyproline | |||
| content (µg/g) | ||||||
| Normal | -- | 127.73 ± 10.65 100.26 ± 11.50 40.11 ± 2.20 | 0.11 ± 0.02 | 235 ± 20 | ||
| 1.82 ± 0.08 | ||||||
| Vehicle + CCl4 | -- | 1262.30 ± 1.97 903.50 ± 30.00 104.09 ± 3.00 | 955 ± 13 | |||
| S. mukorossi + CCl4 | ||||||