1.
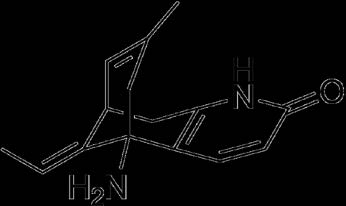
In literature, several methods have been described for analysis of Losartan potassium in API and formulations. Various methods are HPLC based [1,2], (CE) capillary electrophoresis [3], voltametric determination [4] and some are spectrophotometric [5][6][7].
But there is no single analytical method have been reported for determination of losartan as simple and economical like this method. Because I have used simple water for analysis of these all brandsand in very less time period I have analysed the drugs.we have done this types of assay for other drugs which will be useful for small scale laboratory and where expensive Author: Faculty of Pharmacy, Jinnah University for Women, Karachi, Pakistan. e-mails: [email protected], [email protected] instrument not available we can easily find out these drugs in a very short period of time. The serial number as an identification of purchased brands are given in Table 1. Using 20 tablets of six different brand of losartan from the marketed sample were weighed and average mean were calculated. By calculating the average weighed powder of each brand equivalent to 10 mg of losartan was transferred in a volumetric flask containing small water then solution was sonicated for about 5 min and than make up volume upto 100 ml with water.Same procedure was repeat for all brands for preparation of solutions.
2. e) Procedure
After preparation of standard and sample solutions of different brands, strength of all solutions 100 ppm in 100 ml. By using 234 nm wavelength absorbance noted and calculate % assay of each drug.
3. III.
4. Results and Discussions
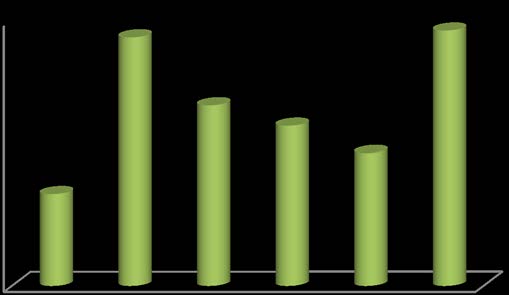
Pharmaceutical assay was carried out by using spectrophotometer on six brands of losartan tablets.
5. Conclusion
A simple, rapid, and economical UV method has been established for determination of losartan alone or in their formulations. This method has several advantages, including simple sample preparation and rapid analysis. It is suitable for analysis of antihypertensive agent losartan in their formulations in a single run, in contrast with previous published methods. This makes the method suitable for routine analysis in QC quality-control laboratories.

| Absorbance at | |||||
| Brand Name Code Average wt of tablet mg | Wt for 100 ppm | 234 nm | % assay | ||
| AZA | LSR1 | 0.16 | 0.016 | 2.627 | 101.0385 |
| cozaar | LSR2 | 0.156 | 0.015 | 2.673 | 102.8077 |
| losaan | LSR3 | 0.153 | 0.015 | 2.653 | 102.0385 |
| zostat | LSR4 | 0.18 | 0.018 | 2.647 | 101.8077 |
| losark | LSR5 | 0.234 | 0.023 | 2.639 | 101.5 |
| eziday | LSR6 | 0.175 | 0.017 | 2.675 | 102.8846 |