1. Introduction
lobally about 34 million people were living with HIV in 2012 1,2 . Still, there were about 2.2 million new infections 3 . Since the beginning of the epidemic nearly 30 million people have died of AIDSrelated causes 1,2,4 .
At end of 2010 about 22.9 million which is 67% of those living with HIV/AIDS globally are in Africa though only about 12% of the world's population lives in the region 2 . In terms of mortality, the region represents about 79% of AIDS mortality globally 5 , the estimated mortality from AIDS related illnesses at end of 2010 are 1.2 million 2 .
Author: Debre Markos University, College of medicine and health science, public health department. e-mail: [email protected] According to 2011 Ethiopian demographic health survey HIV prevalence in Ethiopia was 1.5 % and in the study area of Amhara region, it was 2.2% 6 .
In Ethiopia the fee based and universal free access Antiretroviral (ARV) treatment was started in 2002/3 and 2004/5 respectively. The country uses decentralizing the ARV treatment service provision to the level of Health centers and private Health facilities for fast expansion of the service 7 .
The major causes of morbidity and mortality of HIV/AIDS patients are OIs 8 that would occur in up to 40% of PLWHA with a CD4 count less than 250/mm3 9 .
In North India, TB was the commonest OI (71%) followed by candidiasis (39.3%), PCP (7.4%), cryptococcal meningitis and cerebral toxoplasmosis (3.7% each) 10 .
A national study in Ethiopia showed HIV patients' had OIs like Herpes Zoster scar (19.3%); pulmonary tuberculosis (5.2%) and pneumonia (5.2%) and some patients (2%) had more than one neurologic complications of HIV/AIDS 11 . In Northwest Ethiopia about 7.5% and8.3% of the HIV patients' had pulmonary tuberculosis and Cryptococcal meningitis respectively 12,13 . Nearly a quarter (22.7%) of HIV patients' had chronic diarrhea in Southern Ethiopia 14 .
Even though, OIs are prevalent in the study area there is no local evidence on time gap between repeated re-happening OIs after prior treatment among PLWHA who are initiated ART. Thus the current study would give OI free time and its associated factors that can be used to plan resources and to identify PLWHA who need especial care. The evidence is expected to be used by governmental and non-governmental organizations working on HIV/AIDS. In the town there is one referral hospital and one health center that provide chronic HIV care. All 18 years old and above PLWHA who develop OI after 30 days of starting ART (the first 30 days after HAART were excluded due to most immune reconstitution inflammatory syndrome occurring in the period 15 ) and taking standard treatment according to the Ethiopian Ministry of Health guideline were the study populations. HIV patients who take treatment for OI but not returned at least once to health institution for follow up; those who did not develop any OI since registered on HIV care after starting ART; and their follow up format incompletely documented when treatment for OI given or on consecutive follow ups were excluded from the study.
2. II.
3. Methods and Materials
4. b) Sampling and data collection procedure
The sample size was calculated based on the assumption of 95% confidence interval and 2.5% of absolute precision and the proportion of pulmonary tuberculosis (6%) among PLWHA who are initiating ART 16 . The calculate sample size using Open Epi Version 2.3, May 2009 was 347 and after adding 5% contingency the final sample size was becoming 364.
After preparing the sampling frame among PLWHA commencing ART that fulfill the inclusion criteria, selection of study participants were done using simple random sampling technique via random number table method.
Data collection instrument was developed from both federal ministry of health chronic HIV care form and the patient's card in which the follow up health status data were registered. Then the needed data was collected by reviewing ART follow up form, laboratory request and patients' card. If laboratory examinations like CD4 count, Hemoglobin, weight are not found during entry and exit to the study, the measurements that are most nearest to time of entry and exit to study were taken as baseline and end line predictors respectively.
Participants whose future time re-happening of OI not confirmed due to loss follow-up/dropout/transferred out/dead by any disease other than OI/cause of death not confirmed during study period or not develop OI at end of the study period were censored.
Health professionals working on ART clinics were collecting the data after taking appropriate training on objective of the study and about the data collection instrument in detail. iii. Censored: None re-happening of OI in study participant during follow up on study; but future re-happening is not certain. iv. Drop out: if PLWHA on HIV care lost to follow-up above 3 months as recorded by health personnel working on ART clinic.
v. Lost to follow-up: if PLWHA on HIV care not seen for >1 month as recorded by health personnel working on ART clinic. vi. Transferred-out: if PLWHA on HIV care in one health institution shift to other health institution.
vii. Good Adherence: if PLWHA adherent > 95 % that is the percentage of missed dose is < 2 doses of 30 doses or <3 dose of 60 dose) as documented by health personnel working on ART clinic.
viii. Fair Adherence: if PLWHA adherent 85-94 % that is the percentage of missed dose is 3-5 doses of 30 doses or 3-9 dose of 60 dose) as documented by health personnel working on ART clinic.
ix. Poor Adherence: if PLHIV adherent <85% that is the percentage of missed dose is > 6 doses of 30 doses or >9 dose of 60 dose) as documented by health personnel working on ART clinic.
5. d) Statistical Analysis
A coded questionnaire was double entered in to Epi Info version 3.5.1 statistical package by a trained data clerk and exported to SPSS version 20 and STATA version11 statistical packages for analysis of statistical inferences. Before further analysis, data cleaning was done using frequencies, cross tabulations, sorting and listing to check missed values and outliers. Errors identified during the process were corrected by revising the original questionnaire.
To estimate the time of OI free duration, the actuarial life table and Kaplan Meier survival was used. Assumption of proportional-hazard was checked using Schoenfeld residual with p-value >0.1(?=10%) and the assumption was not violated. Base line and end line hemoglobin value was correlated (r=0.48, p=0.006) thus end line hemoglobin value was excluded from multivariate analysis due to affecting the final model by its redundancy nature which affects precision of estimate. The hazard rate at uni-variate and Multivariate level was calculated using Cox proportional-hazard model. Variables having p-value <0.05 at uni-variate analysis and not collinear was entered into final model of multivariate analysis to identify associated factors with outcome. clinics prepared the sampling frame and extracted the data from medical records. In addition no personal identifier was extracted on medical records.
6. III.
7. Result
In the six year follow up period majority of the participants were females (64.6%), orthodox Christians (91.6%), living in urban (74.5%), married (46.4%), not educated (41.5%) and not employed (74.2%) in governmental or private sectors. Their median age was 32 years in which all most all of them were below 50 years old (table 1).
The base line and end line CD4 count values were 159 and 313 cells/ul respectively. The respective base line and end line mean values for hemoglobin were 11.9 (±2.5) and 12.4 (±1.9) g/dl and for body mass index it was 18.9 (±3) and 19.7 (±2.9) kg/m 2 . At start of the study about 72% of the participants were diagnosed only one type of OI while the rest was diagnosed 2 or more OIs at one visit of health institution. Of the diagnosed OIs at start about half (51.1%) was having WHO stage III OI. All most all (98.4%) the participants have no other concomitant chronic diseases like hypertension, cardiac disease, and diabetes mellitus. Nearly all study subjects were having working functional status both at base line (71.7%) and at follow up (89%). All participants were on first line ART regimens in which about 40.4% and 56.9% were taking Tenofovirdisoproxilfumarate+ Lamivudine+Efavirenzregi men both at base line and at end line respectively and their drug adherence status was good both at base line (94.8%) and at follow up (93.7%). Most of the study subjects were taking Prophylaxis is both at base line (93.1%) and at follow up (92.3%) and their drug adherence status was good both at base line (95.7%) and at follow up (94.5%) (Table1).
8. a) Time gap of OI re-happening and associated factors
During follow up the cumulative incidence of OI re-happening was 76.9% (95% CI: 72.6-81.25) and the incidence rate was 1.1 (95% CI: 0.97-1.23) per 100 person weeks. The commonly re-happening OIs were recurrent upper respiratory tract infection (19.3%), bacterial pneumonia (12.1%), oral candidiasis (10.4%), chronic diarrhea (9.3%), herpes zoster (9.3%), pulmonary tuberculosis (6.1%), extra pulmonary tuberculosis (7.1%), PCP (3.9%), encephalopathy (3.9%), toxoplasmosis (3.2%), and other types (3.3%).
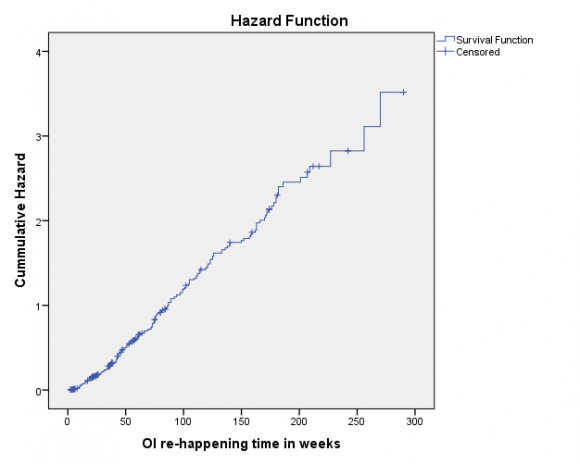
According to Kaplan Meier survival estimation, the median time of OI re-happening was 66 (95% CI: 57.87-74.13) weeks (figure 1). As the actuarial life table analysis showed the probability of free of OI rehappening with in the first five weeks was 97% and it was becoming <10% and <1% after 180 and 255 weeks respectively.
After adjustment for potential confounders in multivariate cox proportional hazard model, the factors that delay re-happening of OIs were being educated than non-educated, taking Prophylaxisis at follow up, having a hemoglobin level above 10 g/dl at base line, having a CD4 level above 100 compared <100 cells/ul both at base line and at end line. But being widowed compared to married and not adhering ART drug at base line were risks for short time re-happening of OIs (Table 1). IV.
9. Discussion
In current study, the cumulative incidence of OI re-happening was 76.9% and the commonly rehappening OIs were recurrent upper respiratory tract infection (19.3%), bacterial pneumonia (12.1%), oral candidiasis (10.4%), chronic diarrhea (9.3%), and herpes zoster (9.3%). And this finding was nearly in agreement with prior studies 9,10,[12][13][14]16 though some figures are slightly vary among prior findings each other and with the current study due to difference in study design (prior ones are cross sectionals), and study area which is conducted in various socio-economic characteristics.
With regarding sex various studies having contradicting outcome as risk for OI. In a cohort study, female sex increases the risk of toxoplasmic encephalitis 17 . A cohort study in United states showed female gender, were associated with significantly higher odds of OIs like herpes simplex virus-2 infection 18 . In contrary, a study in Thailand and showed male gender was significantly associated with higher incidence of OIs after ART 19 . In current study sex is not significantly associated and the possible reasons might be vary in study population, study design and differences in sociocultural contexts of the source population.
One of the factor that delay OI re-happening in current study was having a CD4 count above 100 compared <100 cells/ul both at base line and at end line and this finding was in conformity with other studies 17,[20][21][22][23] . The HIV cohort study in Switzerland showed the baseline CD4 count is one of the predictor for OI progression 20 . Another cohort study also showed higher CD4 cell count was associated with a reduction of risk of new OI progression 23 .
The current finding shown as exposure for prophylaxis at follow up would delay repeated rehappening of OIs and this is in supported by other studies 21,[24][25][26][27] . Primary prophylaxis with Trimethoprimsulfamethoxazole is preventing OIs 24 . Cotrimoxazole prophylaxis prevents diarrhea among PLWHA after ART initiation 26 .
In current study not adhering ART drug at base line was the risk for short time re-happening of OIs and the result was supported by two studies 12,28 in which non-adherence of ART was the risk of failure the drug which enhances OI spread.
10. V. Conclusion and Recommendation
OIs were re-diagnosed in majority of participants. In each week the probability of getting the re-happened OI was 1.1 per 100 persons. The median duration of staying free of OI re-happening was 66 weeks. Participants who were educated, taking Prophylaxisis, having a hemoglobin level above 10 g/dl, having a CD4 level above 100 compared <100 cells/ul would not visit heath institutions due to re-happened OI illness on short periods. Whereas those who were widows compared to married and not adhering ART drug would visit heath institutions due to re-happened OI illness on short periods.
11. VI. Acknowledgment
First of all, my deepest gratitude goes to Professor Getnet Mitkie, Dr Alemayehu Worku and Dr Fikre Enquselassie for their continuous and unreserved supports on completion of the work. In addition I would like to thank data collectors and supervisors and the managers of health institutions for giving permission to conduct the study.

| Variables | Diagnosis of Re-happening | Median | CHR (95% CI) | AHR (95% CI) | |
| OI | KMS | ||||
| No (%) | Yes (%) | ||||
| Marital status | |||||
| Married | 45(53.6) | 124 (44.3) | 73 | 1 | 1 |
| Single | 13(15.5) | 48(17.1) | 79 | 1.15(0.82-1.60) | 0.71(0.42-1.21) |
| Divorced | 18(21.4) | 76(27.1) | 72 | 1.11(0.83-1.49) | 0.81(0.52-1.27) |
| Widowed | 8(9.5) | 32(11.4) | 35 | 2.12(1.43-3.14) | 4.65(2.13-10.16) |
| Educational status | |||||
| Not educated | 21(25) | 130(46.4) | 61 | 1 | 1 |
| Grade 1-8 | 19(22.6) | 82(29.3) | 54 | 0.76(0.57-1.0) | 0.78(0.47-1.28) |
| Grade 9-12 | 21(25) | 53(18.9) | 75 | 0.62(0.45-0.87) | 0.25(0.13-0.48) |
| Above grade 12 | 23(27.4) | 15(5.4) | 170 | 0.38(0.22-0.64) | 0.14(0.05-0.43) |
| Occupational status | |||||
| Un-employed | 47(56) | 223(79.6) | 56 | 1 | 1 |
| Employed | 37(44) | 57(20.4) | 92 | 0.61(0.46-0.82) | 0.85(0.51-1.41) |
| WHO staging B. | |||||
| I | 3(3.6) | 22(7.9) | 72 | 1 | 1 |
| II | 30(35.7) | 77(27.5) | 85 | 0.49(0.31-0.79) | 0.80(0.35-1.80) |
| III | 42(50) | 144(51.4) | 61 | 0.53(0.33-0.83) | 0.52(0.23-1.16) |
| IV | 9(10.7) | 37(13.2) | 47 | 0.61(0.36-1.04) | 0.32(0.098-1.01) |
| Prophylaxisis | |||||
| exposure B. | 4(4.8) | 21(7.5) | 33 | 1 | 1 |
| No | 80(95.2) | 259(92.5) | 72 | 0.48(0.31-0.75) | 0.24(0.08-0.69) |
| Yes | |||||
| Prophylaxisis | |||||
| exposure F. | 5(6) | 23(8.2) | 21 | 1 | 1 |
| No | 79(94) | 257(91.8) | 69 | 0.61(0.40-0.94) | 0.41(0.16-1.03) |
| Yes | |||||
| Prophylaxisis | |||||
| adherence B. | 84(100) | 247(94.3) | 72 | 1 | 1 |
| Good | 0(0) | 6(2.3) | 67 | 1.0(0.45-2.26) | 0.21(0.05-1.0) |
| Fair | 0(0) | 9(3.4) | 35 | 3.37(1.71-6.64) | 3.62(0.32-41) |
| Poor | |||||
| Prophylaxisis | |||||
| adherence F . | 82(97.6) | 244(93.5) | 72 | 1 | 1 |
| Good | 2(2.4) | 6(2.3) | 45 | 2.30(1.01-5.23) | 3.44(0.91-12.97) |
| Fair | 0(0) | 11(4.2) | 35 | 3.42(1.85-6.32) | 4.48(0.98-20.50) |
| Poor | |||||
| ART adherence B. | |||||
| Good | 80(95.2) | 265(94.6) | 71 | 1 | 1 |
| Fair | 4(4.8) | 7(2.5) | 42 | 1.71(0.81-3.64) | 15.35(3.12-75.55) |
| Poor | 0(0) | 8(2.9) | 24 | 4.18(2.05-8.53) | 4.21(0.39-45.83) |
| ART adherence F. | |||||
| Good | 83(98.8) | 258(92.1) | 72 | 1 | 1 |
| Fair | 1(1.2) | 9(3.2) | 41 | 2.32(1.19-4.53) | 0.97(0.34-2.65) |
| Poor | 0(0) | 13(4.6) | 24 | 3.69(2.1-6.50) | 1.04(0.16-6.80) |
| CD4 count (cells/?l) B. | |||||
| <=100 | 9(10.7) | 23(8.2) | 66 | 1 | 1 |
| 101-199 | 19(22.6) | 49(17.5) | 75 | 0.60(0.36-0.99) | 0.29(0.12-0.71) |
| 200-350 | 25(29.8) | 98(35) | 61 | 0.76(0.48-1.20) | 0.60(0.26-1.38) |
| 351-499 | 18(21.4) | 70(25) | 50 | 0.69(0.43-1.12) | 1.30(0.52-3.24) |
| >=500 | 13(15.5) | 40(14.3) | 123 | 0.33(0.19-0.56) | 0.87(0.34-2.26) |
| CD4 count (cells/?l) F. | |||||
| <=100 | 4(10.3) | 14(7.4) | 45 | 1 | 1 |
| 101-199 | 6(15.4) | 26(13.8) | 75 | 0.57(0.29-1.09) | 1.19(0.45-3.15) |
| 200-350 | 12(30.8) | 68(36.2) | 83 | 0.50(0.28-0.90) | 0.87(0.36-2.10) |
| ? | |
| Year 2014 | |
| Based on this study finding, the following recommendations can be forwarded ? Key, B.=base line value F.=follow up value CI=confidence interval KMS=Kaplan Meier survival in weeks CHR=crude hazard rate AHR=Adjusted hazard rate | ( D D D D ) K |