1. I. Introduction
atenin present as ?-catenin (102 kDa), ?-catenin (88 kDa), and ?-catenin (80 kDa) are anchoring proteins present in cytoplasm and verry essential in maintaining the normal functions of E-cadherin protein in the cross-linkage action between actin filament and the intracellular membranous proteins, Na+/K+ adenosine triphosphatase and E-cadherin [1].
?-catenin or alpha-1-catenin (also called alpha-E-catenin) binding protein, is effectively coordinating the cortical actin networks of adjacent cells [2], Roles for ?cat are best understood at cell junctions, where itis essential for cell cohesion and tissue organization [3][4][5]. Asa homodimer, ?-cat directly interacts with filamentous (F) actin [6] but ?-cat can also indirectly associate with the cytoskeleton through other actin-binding proteins, such as epithelial protein lostin neoplasm (EPLIN) (figure 1) [7][8], vinculin [9], afadin [10], ?-actinin [11], and zonula occludens-1 (ZO-1) [12]. In addition, ?-cat can impact F-actin remodeling by directly inhibiting Arp2/3-mediated actin polymerization in vitro [13], lamellipodial dynamics in cells [14],and by promoting Factin bundling in vitro [15].
?-Catenin might serve as an invasion suppressor molecule, and reduced expression of ?catenin has been related to poor differentiation of tumours, infiltrative growth, and lymph node metastasis [16][17][18].
Furthermore, the disappearance of membranous ?-catenin is predictive of an unfavourable outcome in prostate, ovarian, and colorectal cancer [19][20][21]. many studies have shown that ?-catenin represses the transcriptional activities by segregating the YAP1/TAZ transcriptional coactivator in inactive complexes within the cytoplasm [22] According to World Health Organization, carcinoma of oral cavity in males in developing countries, is the sixth commonest cancer after lung, prostrate, colorectal, stomach and bladder cancer, while in females, it is the tenth commonest site of cancer after breast, colorectal, lung, stomach, uterus, cervix, ovary, bladder and liver [23]. More than 90% of all oral cancers are squamous cell carcinomas (SCC) [1]and this type of cancers composes About 95% of oral cancers in India [24].This malignancy constitute a major health problem in developing countries, representing a leading cause of death. The survival index continues to be small (50%), as compared to the progress in diagnosis and treatment of other malignant tumors [25].
This study aimed to show the relation between altered expression of ?-catenin and the histopathological differentiations of oral squamous cell carcinoma.
2. II. Materials and Methods
81 Formalin-fixed, paraffin embedded representative tissue sections 3?m in thickness of 30 well Sections were dewaxed, rehydrated in graded alcohols, and immunostained using a standard streptavidin-biotin immuno-peroxidase method. Monoclonal antibodies against ?-Catenin (RB-089-P, 1:5 dilution, Neomarkers, USA) were used.
Normal oral epithelium was used as a positive control and sections incubated with a negative control serum (Dako, Denmark) were used as negative controls. Immunostaining was evaluated according to the intensity (slight/ strong) and the distribution of staining pattern (homogenous-membranous; heterogenouscytoplasmic and/or membranous).
Immunostaining pattern was scored as follows: 0= nostaining, +1= heterogenous slight staining, +2= homogenous strong staining with respect to the control positive tissue. The intensity and the staining pattern in normal oral squamous epithelium were regarded as +2 homogenous strong staining.
3. a) Statistical analysis
The chi-square test was used to assess the statistical significance of ? -Catenin expression in relation to histopathological grade.
4. III. Results
5. a) Normal epethelium
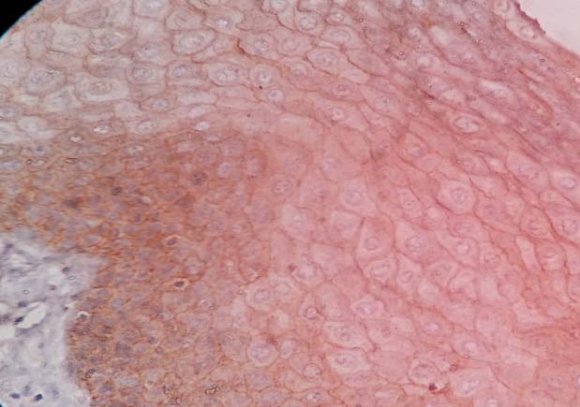
?-catenin staining was cytoplasmic with a clearly strong intensity and showed homogenous strong membranous staining in basal, parabasal and intermediate layers of squamous epithelium of the normal tissues (figure 2 ).
6. b) study sample
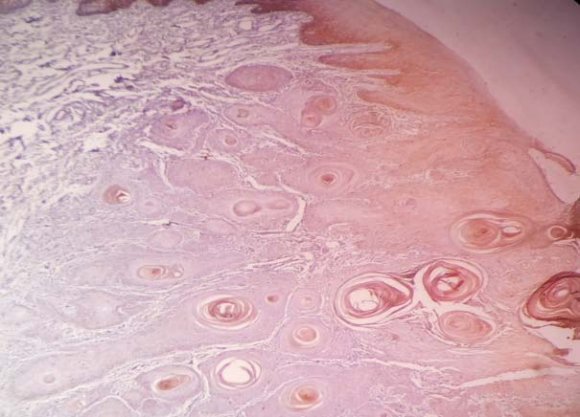
homogenous strong positivity appeared in 56.6% of the WDOSCC sections in the epithelium and the tumoral islands, 40% revealed +1 and in one section we noticed that there was no staining (figure 3). MDOSCCC revealed 40%(+2) immunostaining, 52% (+1) and two showed no staining (figure 4). PDOSCC had only 4 (15%) strong immunostaining (figure 5) (p=0.0001). Aberrant nuclear staining of ?-catenin was observed in a few cells of PDOSCC. (table 1) (figure 6)
7. IV. Discussion
Loss of cell adhesion molecules or altered expression of these molecules plays an essential role in tumor progression in epithelial tissues [26]. E-cadherin and its associated cytoplasmic protein ?-catenin are of the main parts of cell adhesion complex in squamous epithelial tissues [27].
We investigated the expression ?-catenin in oral squamous cell carcinoma progression from the well differentiated stage to the poorly differentiated.
Though we revealed ?-catenin expression loss in the progression of squamous cell carcinoma, this reduced expression was clearly associated with the histopathological differentiation (p<0.05). revealed such loss of ?-cateninin the cases of oral squamous cell carcinomas [28,29] Unlike ?-catenin, which has a role as an oncogene [30], ?-catenin is considered a potent suppressor in many tumors, and its loss or downregulation in many aggressive cancers is clearly correlated with metastasis [31,32]. In addition to its well-known role in cell-cell adhesion, ?-catenin represses signaling through the Wnt, Ras, NF-kB, and Hedgehog pathways [33] which controls organs sizes and cell contact inhibition by way of the Yes-associated protein YAP1. YAP1 is a potent coactivator in many signaling pathways and also interacts with ?-catenin in TBX5 complexes to regulate anti-apoptotic genes in colon cancer [34] At high cell density, phosphorylated YAP1 accumulates in the cytoplasm, where it is sequestered by ?-catenin and inhibits Wnt signaling [22] . The YAP1 homolog TAZ is degraded by the APC complex and is required for expression of many Wnt target genes [35] Mechanistic studies of YAP1 function in TGFb/SMAD signaling further reveal that it both stimulates transcription and promotes the exchange of coactivator and corepressor complexes at target genes [36] Thus, ?-catenin links cell adhesion signals to YAP1 inactivation and the inhibition ofcell proliferation.
?-catenin may potentially control TAZ functions directly at Wnt target genes or guide it to the cytoplasm for degredation by the APC complex. Because ?-catenin and APC are recruited with ?-cateninto target genes, their transcriptional activities must be under control to prevent premature termination of transcription. [37,38]

![Figure1: Cell adhesion Complex. E-cadherin is stabilised at the cell surface by its link to the actin cytoskeleton via ?-catenin, ?-catenin, and, possibly, Epithelial Protein Lost in Neoplasm (EPLIN).[7]](https://medicalresearchjournal.org/index.php/GJMR/article/download/100579/version/100579/2-Catenin-Role-and-Expression_html/9757/image-3.png)
| ?-Catenin Role and Expression in Oral Squamous Cell Carcinoma | |
| carcinoma: a meta-analysis. Asian Pac J Cancer | |
| Prev, 2014. 15(15): p. 6103-8. | |
| 31. Sun, Y., J. Zhang, and L. Ma, alpha-catenin. A tumor | |
| suppressor beyond adherens junctions. Cell Cycle, | |
| 2014. 13(15): p. 2334-9. | |
| 32. Piao, H.L., et al., alpha-catenin acts as a tumour | |
| suppressor in E-cadherin-negative basal-like breast | |
| cancer by inhibiting NF-kappaB signalling. Nat Cell | |
| Biol, 2014. 16(3): p. 245-54. | |
| 33. Schlegelmilch, K., et al., Yap1 acts downstream of | |
| alpha-catenin to control epidermal proliferation. Cell, | |
| 2011. 144(5): p. 782-95. | |
| 34. Rosenbluh, J., et al., beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. | Year 2015 |
| Research ( ) C | |
| Global Journal of Medical | |
| . | |
| One interesting possibility is that Y177 phosphorylation | |
| © 2015 Global Journals Inc. (US) |