1. Introduction
opper is essential trace element that has the catalysis of a wide range of enzymatic activities, including those involved in the processes of energy production such as cytochrome oxidase, the cell response to oxidant injuries of Cu-Zn superoxide dismutase(SOD). In healthy human adults, the necessity ion is reduced to Cu + and then carried into cells by various transmembrane transporters. Copper and zinc are essential for optimal innate immune function and susceptibility to bacterial infection 1 .In the blood, the major copper carrying proteins is ceruloplasmin 2 and the rest of copper is transported by albumin and histidine 3 .Formation of new blood vessels by a tumor enable tumor growth, invasion, and metastasis facilitates easily to occur. Then, organic chelators of copper can passively reduce cellular copper and serve copper has been shown to inhibit angiogenesis in a wide variety of cancer cell and xenograft system 4 . Antiangiogenic strategies of blood vessel for performed 4 , in which are the embryological formation of new blood vessels, the remodeling of an existing artery to increase its cross-section in response to increased blood flow, and the budding of new capillary branches from existing blood vessels 4 .The progenitor cells migrate to sites of vascularization and differentiate into endothelial cells, forming the vascular plexus.
Especially, copper has been suggested as an important co-factor for angiogenesis 5 . It is also a major copper ion that having been found in variety of tumor tissues and copper-mediated tumor proteasome inhibition 6 .Several clinical trials using copper chelation as either an adjuvant or primary therapy have been conducted.Copper can influence the major stages of tumorigenesis-initiation, promotion, progression, significant role for autophagy of anticancer immunity and immunogenicity,autophagy of tumor antigen, and autophagy in cancer immunotherapy based on preclinical references 7 .Further, copper dependent oxidative damage can be prevented by chelation with the antioxidants dipeptides which with imidazole ring chelate copper.As cancer cells exist probably under significant oxidative stress, the cytotoxic levels could be a successful anticancer approach, in which leads to ROS ( O 2 to H 2 O 2 ) and oxygen by generations of copper-zinc SOD enzymes 8 .
On the other hand, cancer is one of the leading causes of mortality and represents a tremendous burden on patients and societies.Colorectal cancers are associated with one of the highest morbidity and mortality rates in both men and women.Cancer arises from a single cell, in which malignant tumors are described as monoclonal, meaning that each tumor arises from a single cell. Cancer cells are characterized by increased proliferation and reduced apoptosis.The development of a malignant tumor from a normal cell usually inhibitions forthe driving force in cancer progression may be various molecular such as Tumor microenvironment 11 , K-ras mutations 12 , and Haplo-insufficiency 13 as a driving force are new findings highlight to investigate cancer invasion and metastasis.Recently, it is worth noting that copper chelation 14 and Cu-polymer compounds 15 kill the cancer cells with copper-binding protein formations 16 .Thus, copper is a vital mineral essential for many biological processes, in which copper also plays an important role in promoting physiological and malignant angiogenesis.Copper deficiency as an anti-cancer strategy is that in an early(phase?) clinical trial have led to ongoing phase?evaluation of the copper chelatorTetrathiomolybdate(TM, as an anti-angiogenic agent) in patients with advanced cancers 17 . The TM may be most beneficial for patient with minimal disease burden in the metastatic setting, in which ongoing phase studies as well as future trials will attempt to exploit this knowledge to define the role of TM in cancer treatment. The other, the vast majorityof all Cu in healthy humans is associated with enzyme prosthetic groups or bound to proteins.Excess or toxicity of Cu, which is associated with the pathogenesis of hepatic disorder, neurodegenerative changes and other disease condition, can occur when Cu homeostasis is disrupted 18 .
In this review, it has becoming revealed on the standpoint of the results obtained from Cu 2+ ion killing mechanism against bacteria whether Cu 2+ ions and its compounds may be directly suppressed against the cancer and tumor cells.
2. II.
3. Bacteriolysis of s.aureus pgn and e.coli Outer Membrane Cell Walls
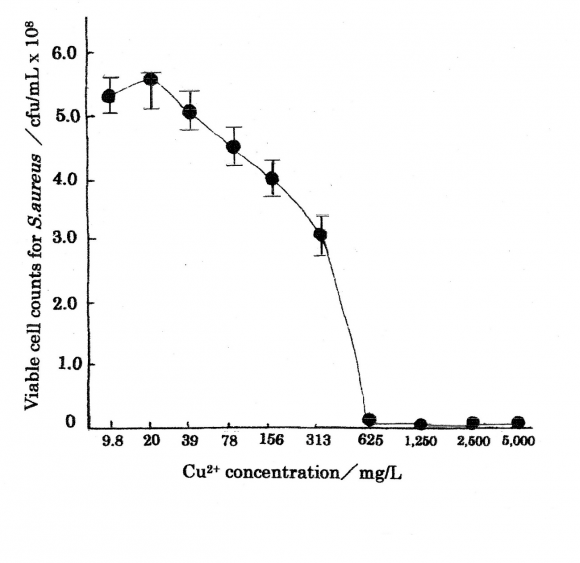
Cu 2+ ions are important as antibacterial agents cells. Table 1 shows the bacteriostasis as disinfection agent inhibiting the bacteria growth and multiplying organism of Cu 2+ ion, in which minimum inhibitory Cu 2+ ion concentration range of 0.10?50 mg/L against E.coli 19 .Table 2 indicates the results as bactericide action, in which MIC=625 mg/L and minimum bactericide concentration, MBC=1250 mg/L were obtained for Cu 2+ ion concentration range of 9.8?5000 mg/L against S.aureus 20 . The killing curve of Cu 2+ ions is shown in Fig. 1 (measurement's error=±6%), in which killing effects for the copper(?) ions appear sufficiently. Killing mechanisms of Cu 2+ ion solutions against bacteria are outlined below.?Bacteriolysis of S.aureus peptidoglycan(PGN) cell wall by Cu 2+ ions is ascribed to the inhibition of PGN elongation due to the damages of PGN biosynthesis; transglycosylase (TG), transpeptidase (TP) and the activations of PGN autolysins. The other,?bacteriolysis of E.coli outer membrane cell wall by Cu 2+ ions is attributed to the destruction of outer membrane structure and to the inhibition of PGN elongation due to the damage of PGN biosynthesis TP 21 and the activations of PGN autolysins 22 .
4. Cancer Development and Progression
Cancer process is comprised of initiated cancer, development and progression of cancer, proliferation, invasion, and metastasis.Progression process of cancerous changes is considered for the cancer and tumor cells in the following: ? Abnormal cell generation ? ? Formation of malignant cell and growth ? ? ? Dedifferentiation and stage of propagation in single cell.
Copper becomes an essential cofactor for cancer cell proliferation, differentiation, invasion, and metastasis,and apoptosis and necrosis.
Carcinogenesis follows the activation of oncogenes and the deactivation of tumor suppression genes.Apoptosis is highly regulated process of cell death in the development and maintenance of a normal cell population in mature organism. Deregulation of apoptosis pathways is thus a key feature of promotion, malignant cell,cell invasion and metastasis against cancer and tumor cells.
5. a) Cancer prevention and initiated process
Clioquinol(CQ)-CuCl 2 mixture 23 indicates a formation of a stable CQ-Cu complex, and 1,10phenanthroine 24 promotes copper complexes into tumor cells and induces apoptosis by inhibiting the proteasome activity.
Catechins, the dietary phytochemicals present in green tea and other beverages, are considered to bepotent inducers of apoptosis and cytotoxicity to cancer cells, in which the antioxidant properties make cancer induction lowering and impeding oxidative injury to DNA 25 . The cellular DNA breakage was found to be significantly enhanced in the presence of copper ions. These Cu complexes play role of cancer prevention.
6. b) Promotion
Initiation process: copper(?) ions inactivate
Cu 2+ + -SH ? -SCu(?) + H +Oxygen in the cell varies reductive superoxide anion, that generates hydrogen peroxide. Volume XVII Issue VI Version I
O 2 + e -? O 2 - 2O 2 -+ 2H + ? H 2 O 2 + O 2 O 2 - ? H 2 O 2 ? OH -+ ?OH + O 2 - O 2 + e -+ H + ? ?HO 2 ?HO 2 ? H + + O 2Year 2017 ( D D D D ) K Cu 2+ + O 2 - ? Cu + + O 2 Cu + + H 2 O 2 ?Cu 2+ + ?OH + OH - c) ProgressionProgression of cancer or tumor cell is carcinogenesis, oncogenesis, epigenesis 26 and the migration for intercellular ion channels 27 are focused on the identification. Epigenetics in carcinogenesis, progression, and metastasis occurring from cancer stem cell have investigated that many epigenetic changes such as hypomethylation of oncogenes, hypermethylation of tumor suppressor genes, are known to be associated with many cancers. The other, the intracellular ion channels have emerged as oncogenic proteins, since they have an aberrant expression in cancers compared to normal tissues and contribute to several hallmarks of cancer. Carcinogenesis follows the activation of oncogenes and the deactivation of tumor suppression genes. Cu 2+ induced initial cancer cell ROS production and oxidative stress against tumor cell 28 .
In free radicals (O 2 -, H + , OH -,?OH) and H 2 O 2 are formed as follows 29 :
?O 2 -+ 2H + + e -? H 2 O 2 H 2 O 2 + e -? HO -+ ?OH ?OH + e -+ H + ? H 2 O 2H + + ?O 2 -+?O 2 -? H 2 O 2 + O 2 H 2 O ? ?OH + ?H + e -? H 2 O 2In the cell wall, reacting with polyunsaturated fatty acids(L=Organic ligand).:
LH + OH? ? L? + HOH L? + O 2 ? LOO? LH + LOO? ? L? + LOOH Haber-Weiss reaction 30 ; H 2 O 2 + O 2 -? ?OH +OH -+O 2 Fenton reaction 31 ; Cu + ?H 2 O 2 ? ?OH ?OH -+ Cu 2+Furthermore, new ROS productions occur by Fenton-like type. L=Ligand
LCu(?) + H 2 O 2 ? LCu(?)+ ?OOH + H ? LCu(?)?H 2 O 2 ? LCu(?)??OH + OH -The other, relation of oxidative stress and autophagy has been investigated for copper ion in Cu 2 O, CuO crystals. The aqueous systems as following reaction 32 :
Cu 2 O + 2H + ? 2Cu + + H 2 O and CuO + 2H + ? Cu 2+ + H 2 OCu + ion is unstable and easily oxidized to Cu 2+ ion in aqueous system by Fenton reaction. Hence, in blood it is not proper as Cu + ion rapidly is oxidized to Cu 2+ ion. However, although "self-eating" by autophagy can potentially lead to cell death when cytoplasmic cellular organelles are consumed beyond a critical-forcell-survival point, it is unclear whether autophagy represent an active dying mode or the cell desperate, and often exhausted, attempt to survive.
7. d) Invasion and metastasis
Cancer cell invasion has collective and individual cell migrations, by which cancer cells invade other tissues either by moving collectively as epithelial sheets or detached cluster, or as single cells via mesenchymal or amoeboid cell types 33 . During cancer progression, a variety of tumor cells show changes in their plasticity by morphological and phenotypical conversions, including the epithelial to mesenchymal transition (EMT). EMT has been increasingly recognized as crucial events in cancer progression and metastasis. Human epithelial cells predominantly migrate collectively, while most cells observed in vivo using intravital techniques and in vitro studies migrate as single cells 34 .
The other, metastasis is a multi-step process encompassing, ? the local infiltration of tumor cells into the adjacent tissue, ? transendothelial migration of cancer cells into vessels known as intravasation, ? survival in the circulatory system, ? extravasation and ? subsequently, proliferation in competent organs leading to colonization 35 . The rate-determining step process is that there is great interest in understanding the regulation of cellular adhesion metal-protein molecule. The epithelial to mesenchymal transition (EMT) is observed phenomenon that is a vital aspect of embryogenesis as well as cancer progression. During the EMT, cancer cells lose their adhesion and begin the process of metastasis ?. The process of cancer cell transition from EMT plays a dominant role in facilitating metastasis and progression in many types of cancer. Cuprous oxide nanoparticle (CONPs) induce mitochondria-mediated apoptosis, indicating that can inhibit the growth and metastasis of cancer cells 36 .
8. IV.
Cu 2+ Peptide copper complex may be formed as 3N-Cu-O, Cu(Gly-L-Ala)H 2 O. Specially,Cu 2+ ions react with such as cross linked molecular penta glycine(Gly) 5 , copper-glycine complex may be formed.
9. b) Autophagy in cancer cell
Autophagy plays an important role in cancer and tumor cells. However, how autophagy contributes to cancer ontogenesis and progression has turned out to be more complex than expected. It must be clear whether Cu 2+ ions induced autophagy or necrotic cell death. Autophagy is to be function as tumor suppression of damaged organelles/proteins, and to confer stress tolerance that can maintain tumor cell, and to be a mechanism of cell death. MCF-7 cells influenced with tested Cu(?) complexes produced LC3 protein after 72 hours incubation indicating autophagy in MCF-7 cancer cells 37 . Further, the specific nanomedicine induced phage fusion protein in cancer cell occur, that has shown significant improvements in the therapeutic activity of currently existing drug delivery system, such as liposomal doxorubicin. Thus, this fact is implicated that in the cancer and tumor cells, the killing modes are elucidated, it must be clear in this study that the inhibitions of progression and development, invasion, and metastasis of tumor cell occur by Cu 2+ induced autophagy fusion proteins in cancer and tumor cells 38 . Furthermore, autophagic anticancer immunity pays attention in which autophagy affects the anti-cancer immune response. Accumulated studies have demonstrated that triggering autophagy is able to facilitate anticancer immunity due to an increase in immunogenicity, whereas other studies suggested that autophagy is likely to disarm anticancer immunity mediated by nature killer(NK) cell.Cu 2 O crystals promote endothelial cell death via Cu + induced autophagy, and elevate the level of reactive oxygen species such as superoxide and nitric oxide 36 .Active role of autophagy as a cell death mechanism can be in principle validated by experiments documenting prolongation of survival upon autography downregulation 36 . However, the endothelial cell death by Cu + ion induced autophagy is unclear whether the tumor death is due to fusion proteins in process of autophagy 38 .
10. c) Cu 2+ ions, copper complexes and copper-chelating suppress tumor development and angiogenesis in the cancer cell
Tumors are to grow and thrive that they must develop a blood supply. Thus, it is said that every increment in tumor growth requires an increment in capillary growth, in which neovascularization or mechanism by that tumor cells elicit new blood vessel growth from the surrounding tissue. Angiogenesis is a complex process with many different growth factor and inhibited by a diverse range of proteins. The molecules secreted by tumors act on stromal cells in a paracrine fashion, so that can have different activities with the production and secretion of antiangiogenic proteins.
Copper is required for high levels of angiogenesis, in which copper requirement is due to many angiogenic factors. Angiogenesis relies on the coordination with many different activities in copper complex and copperchelating for suppressor tumor.
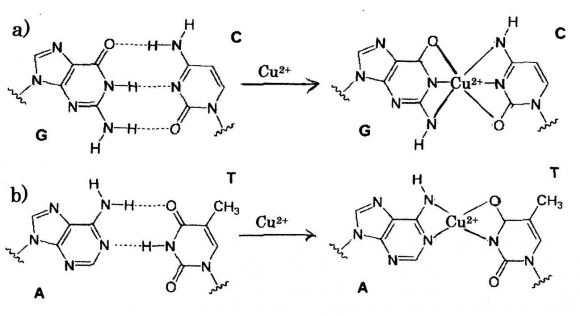
Copper as a neovascular agent is required for angiogenesis, in which micro-molar amounts of Cu(10 -6 M), thus appeared to control endothelial cell migration and angiogenesis. Copper was shown to stimulate blood vessel formation in the avascular cornea of rabbits, only recently have clinical trials established that Cu privation by diet or by Cu chelators diminishes a tumor's ability to mount an angiogenic response 39 .Nanoparticles of copper(NanoCu) stimulate Tetrathiomolybdate(MoS 4 2-,TM) 41 is a very promising antiangiogenic agent, and a potent metal chelator that binds Cu to proteins such as serum albumin, forming a complex that is only sparingly taken up by cells. The underlying concept for TM efficacy as an anticancer agent is that when the copper status is in the window, cellular copper needs are met and toxicity is avoided. Copper deficiency induced TM 42 , depletion of copper 43 and copper-lowering 44 were significantly impaired tumor growth and angiogenesis, encouraging results in canine study of advanced and metastatic cancer. Further, the copper-chelating agents are efficient for Trientine Dihydrochloride (trientine), suppressor tumor development and angiogenesis 10 . 2+ -DNA: Cu 2+ substitution to hydrogen bond in DNA base pairs Cu 2+ ion induced occurrence of generations of ROS and hydrogen peroxide H 2 O 2 in tumor cells damages DNA in tumor, in which formation of DNA angiogenesis at molecular level 40 . NanoCu affect the development of blood vessel and muscles in a different manner than Cu salts, in which have pro-angiogenic properties at the systemic level, to a greater degree than CuSO 4 salt. The other, NanoCu also were confirmed that demonstrating significant effects on mRNA concentration and on mRNA gene expression of all proangiogenic and pro-proliferative genes measured. K damage resulting from a release of catalytic copper and binding of copper to DNA with generation of ?OH radicals, and by reaction of H 2 O 2 with the metal produces the strand breaks in DNA as well as DNA base modifications and deoxyribose fragmentation. It has been found that in aqueous solution coordination of Cu 2+ to the N7 and N1 sites of purine rings is pH dependent and coordination to N7 action tending to bind purine base A(adenine), G(guanine) and pyrimidine base C(cytosine), T(thymine) of nucleic acid bases for individual metals are indicated 45 , depending on acid dissociation constant pK a . According to the theory, it is shown in Fig. 2, that is represented to substituting of Cu 2+ ions into hydrogen bonds in DNA base-pairing G?C and A=T pairs. Thus, it may be considered that DNA damages due to copper complexes formation within DNA base-pairs G?C, A=Toccur in cytoplasm of cancer cell.
11. d) Copper-nucleotide interaction and Cu
12. e) Copper complexes induced the killing, the regulation, the suppressor against cancer and tumor cells
Copper compounds, complexes, and chelation act beneficial for specific malignant tumors. The high against cancer cell of metastatic liver cancer, prostate cancer that supplementing with Cu, DS is highly toxic to cancer cell 46,47 . Anticancer activity is exhibited by copper(?) complex possessing pyridinetype ligands(pyridine, bipyridine, phenanthroline etc.) or such where copper(?) ion is coordinated to phosphine ligands.
13. Casiopeinas, copper coordinated complexes of Cu(N-N)(A-A)NO 3 , (A-A=N-O,O-O))
with perceptible antineoplastic effects on human malignant glioma had been investigated 50,51,52 . The result is that the Casiopenia?-ia significantly inhibited cell proliferation and cell death, inducing autophagy and apoptosis of glioma cells, which correlated with the formation of autophagic vacuoles, over expression of Bax and Bid proteins. New uses for old copper binding drugs 53 is approached to discover new application for a specific cancer cell death inducer, including pro-angiogenic process.
14. Conclusions
Cu 2+ ions have numerous roles in cancer prevention, initiation of carcinogenesis, progression of uncontrolled cell growth, malignant tumor cell growth, invasive growth as malignancy, and metastasis of downregulation of cell adhesion and cell-cell attachment, by Cu(?)/Cu(?) redox reaction cycles and Cu 2+ ion induced ROS productions.Angiogenesis and autophagy play an important role in cancer and tumor cells. Schiff base copper(?) complexes have anti-proliferative activity against cancer cells. Cu 2+ ions play an important role as pro-cancer factor in tumor tissues, especially in tumor angiogenesis,invasion, and metastasis. Cu 2+ ions blood vessel of tumor cell against angiogenesis in cancer.Promotion and development of cancer tissues have been proceeding with homeostatic imbalances of copper, in which can be caused by the uptake of excessive amounts of copper and some genetic
| Cu 2+ solution | Cu 2+ solution concentration(mg/L) | MIC | |||||||||
| agent? original conc | 50 | 25 | 12.5 | 6.25 | 3.13 | 1.56 | 0.78 | 0.39 | 0.20 | 0.10 | 50 mg/L |
| 500 mg/L | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | above |
| (?)?Visible bacterial growth | (-)?No visible bacterial growth | ||||||||||
| Antibacterial agent | Cu 2+ concentration(mg/L) | |||||||||
| Cu(NO 3 ) 2 3H 2 Osolution | 5000 | 2500 | 1250 | 625 | 313 | 156 | 78 | 39 | 20 | 9.8 |
| MIC | _ | _ | _ | _ | + | + | + | + | + | + |
| MBC | - | - | - | + | + | + | + | + | + | + |
| 3.1 | ||||||||||
| × | ||||||||||
| CFU(cfu/mL) | ?10 | ?10 | ?10 | × | ||||||
| 10 2 | ||||||||||
| 10 8 | ||||||||||
| Cu 2+ ions are in turn reduced to Cu (?) ions by which is partial action sites of glycan saccharide chains. |
| superoxide anion O 2 -. The copper (?) ions can reduce L is coordinated molecular. |
| hydrogen peroxide H 2 O 2 to hydroxyl radical ?OH. Cu 2+ + LH ? CuL + + H + |
| CuL + + LH ? CuL 2 + H + |
| Cu 2+ ? 2LH ? CuL 2 ? H + |
| considered that the uncontrolled cell growth for a |
| Year 2017 |
| Volume XVII Issue VI Version I |
| D D D D ) K |
| ( |
| Reactive oxygen species (ROS) O 2 -and H 2 O 2 |
| generated in cell wall permeate into cell membrane and |
| cytoplasm, in which in cell membrane high reactive |
| ?OH and OH -are formed by Haber-Weiss and Fenton |
| reactions. |
| a) Cu 2+ ions binding with amino, peptide, protein of |
| cancer cell tissues |
| Cu 2+ ions inhibit polymerization of glycan |
| chains, to be thought to be forming copper complex in |
| Furthermore, the copper chelation kills the cancer and | tumor growth. Killing of cancer cell is induced via ROS |
| tumor cells, in which an alternative Cu-chelators 10 and | mainly consisting of singlet oxygen, O 2 -,?OH,and H 2 O 2 . |
| chelator 54 could inhibit and suppress neovascularization, | |
| increase of apoptosis in tumor growth, and | ions migration into initiation, progression, proliferation, |
| angiogenesis. Copper chelating complex can serve as | invasion, and metastasis against cancer and tumor |
| anti-angiogenic agent and ROS generators to inhibit | cells. |