1. I. Introduction
minophenols are interesting electrochemical materials since, unlike aniline and other substituted anilines [1][2], they have two groups (-NH 2 and OH), which could be oxidized. Therefore, they could show electrochemical behavior resembling anilines and/or phenols [3][4][5]. An important factor would be the relative position of the amino and hydroxyl group in the aromatic ring. Accordingly, the reported electrochemical properties of the three positional isomers (ortho, meta and para) are strongly different. P-Aminophenol (p-AP) is a well-known compound which, in its simple form, or derivative [6], has been used as redox agent in photography. In neutral media, it is oxidized to complex oligomeric deys that could be used in enzymatic assays [7]. konopelnik et al. [8] have studied the oxidation of P-aminophenol (P-AP) in aqueous solution on SnO electrodes. According with these authors, only the amino group of m-aminophenol undergoes oxidation while the hydroxyl group remains unchanged. Common laboratory-based analytical methods for determining para-aminophenol compounds such as primarily gas and liquid chromatography (HP LC) [9][10][11][12][13], UV-vis spectrophotometry [14][15] and spectrofluorimetry [16] have been reported. The use of enzyme-link ed immunosorbent assay (ELISA) has been studied [17]. However, some sample pretreatment involving separation, extraction and/or adsorption is generally necessary, and this can also be timeconsuming and complex. Electrochemical methods, such as differential pulse polarography (DP P), anodic stripping voltammetry (ASV) and differential pulse voltammetry (DPV), have been widely applied for the determination of pharmaceuticals, dyes, insecticides and pesticides [18][19][20]. In recent years, chemically modified electrodes (CMEs) were used for the voltammetric quantification of various organic and inorganic species after their open circuit accumulation [21][22] Much of the work in this field was directed to exploit the chemical reactivity of the modifier towards a target analyte for electroanalytical purpose. Multitudes of modifying agents were used either as coatings on solid electrode surfaces or dispersed within a conductive matrix. It is noteworthy that this last approach is well suited when using electronically insulating modifiers requiring a direct contact to an electronically conducting substrate as used in connection with electrochemistry. The application of silicates and related mineral materials in electrochemistry is rather recent and was directed to combine their intrinsic properties to selected electrochemical reactions in order to improve the response of the electrode. Modified electrodes are being used frequently in the voltammetric determination of organic compounds because of their efficiency, the selectivity that can be obtained by varying the modifier and the sensitivity which is equivalent to that reached in anodic and cathodic stripping. In doing so, zeolite and silica-modified electrodes were prepared, characterized and applied (sometimes tentatively) in various fields including for example electroanalysis and sensors, electrocatalysis, photochemistry, thin-film technology, fuel cells, molecular recognition. Kauffmann [23] has reported that a carbon paste electrode (CPE) modified with lipids and proteins (enzymes) have potential application in environmental analysis. Recent works, reported in the literature, have shown several applications and electroanalytical methodologies employing glassy carbon electrode as working electrodes [24][25]. Luz and al. [25] constructed a glassy carbon electrode impregnated with a lithium tetracyanoethylenide (LiTCNE) for the determination of para-aminophenol. The oxidation and reduction of this compound has been carried out on a modified glassy carbon electrode using cyclic and DPV [26][27][28]. This study proposed a new modified carbon paste electrode which has been prepared by the Natural Phosphate (NP) for para-aminophenol detection. It has shown a selective preconcentration and quantization of paraaminophenol by cyclic voltammetry (CV). This study has led to the development of a new modified electrode for the determination of para-aminophenol with improved qualities such as simplicity of electrode preparation, wider linear range, and low detection limit (DL), high selectivity and very good stability of modifier. The procedure is based on the oxidation and reduction of para-aminophenol after it was preconcentrated on a carbon paste electrode modified with the clay, under open circuit conditions.
In this work we prepared and characterized the phosphate Natual modified carbon paste electrode, which successfully exploits the favorable mechanical and electrochemical properties of carbon paste electrodes. Also, this study therefore focused on the determination of the effectiveness of the Moringa oleifera in the purification of water contaminated by 4-Aminophenol.
2. II. Experimental a) Reagents
p-Aminophenol and sodium sulfate were of analytical grade and from Aldrich. A natural phosphate (NP) used in this work was obtained in the Khouribga region (Morocco). Stock solutions of p-Aminophenol were prepared by dissolving p-Aminophenol in deionized water. All preparations and dilution of solutions were made deionized water. Provisions were made for oxygen removal by bubbling the solution with azotes gas for about 5 min then the solution was blanketed with azotes gas while the experiment was in progress. For reproducible results, a fresh solution was made for each experiment. Carbon paste was supplied from (Carbon, Lorraine, ref. 9900, French).
3. b) Electrodes preparation
Firstly, the carbon-paste electrode was prepared according the following procedure [29]. The carbon-paste electrode was prepared by mixing the graphite powder with paraffin oil used as a binder.
The mixture was grinding in a mortar agate and then a portion of the resulting composite material was housed in PTFE cylinder. The geometric surface area of the working electrode was 0.1256 cm2. A bare of carbon vitreous inserted into carbon paste provided the electrical contact, and then the NP film is electrodeposited onto carbon paste electrode. The deposit of Phosphate natural on carbon paste electrode surfaces was processed at 20 V. The current was maintained by a galvanostat with a function generator.
4. c) Prepared electrode characterization
All the electrochemical experiments were performed in a standard one-compartment threeelectrode cell. The reference electrode was SCE and the counter electrode was platinum. All electrode potentials were referred to this reference electrode. The working electrode was NP modified carbon paste electrode (NP-CPE).
5. d) Apparatus
Electrochemical experiments were performed using a voltalab potentiostat (model PGSTAT 100, Eco Chemie B.V., Ultrecht, The Netherlands) driven by the general purpose electrochemical systems data processing software (voltalab master 4 software).
6. III. Results and Discussion
7. a) Characterization of prepared electrodes surfaces
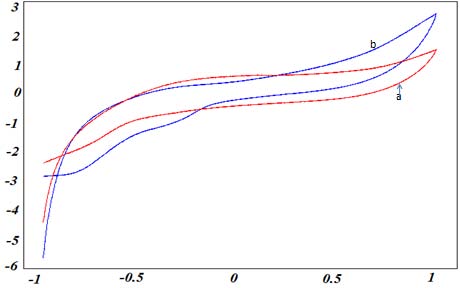
The surface structure of natural phosphate modified carbon paste surface was observed using scanning electron microscopy (Fig. 1). The film layer of NP was formed on the surface of carbon paste electrode; it was not disintegrated or detached from the surface when immersed in the electrolytic solution (0.1M Na2SO4). The morphology of the phosphate rock surface was observed by scanning electron microscopy (Figure 1). The treatment described previously gives compact particle fractions between 100 and 400 µm rich in phosphate. Rock phosphate treaty has the following chemical composition: CaO (54.12%), P 2 O 5 (34.24%), F (3.37%), SiO 2 (2.42%), SO3 (2.21%), CO 2 (1.13%), Na 2 O (0.92 %), MgO (0.68%), Al 2 O 3 (0.46%), Fe 2 O 3 (0.36%), K 2 O (0.04%) and order of several ppm metals. We can see that the shape of the cyclic voltammogram was modified in the presence of NP at CPE surface, suggesting that the carbon paste electrode was effectively modified by natural phosphate.
8. ) Electrochemical detection of studied metals
The experimental conditions have been optimized and the response characteristics determined in a previous work [29]. The results obtained are:
??p H ? ? 7 ??Pre conce ntra tion time = 13 min.
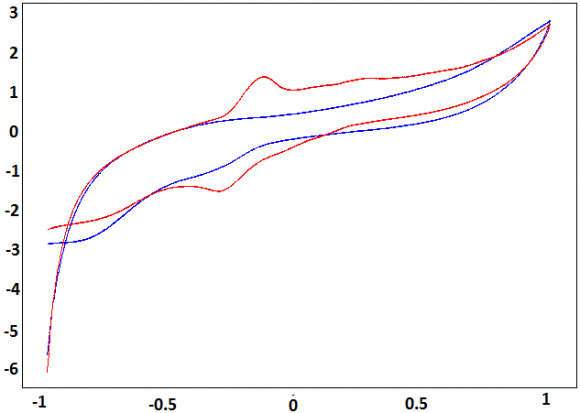
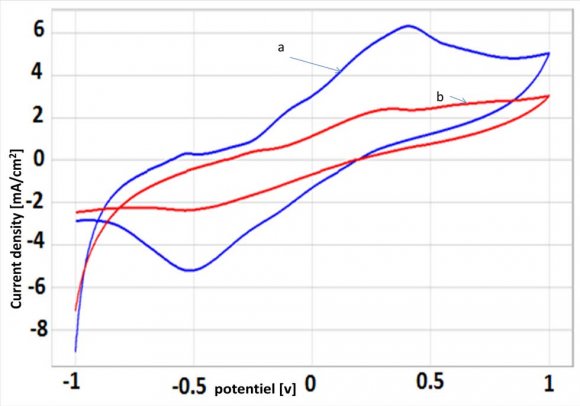
In order to avoid the strong residual of reduction, the starting potential was fixed at -1V versus SCE. Fig. 3 shows a cyclic voltammograms performed between-1 V and 1 V for NP-CPE, in 0.1M Na2SO4 solution (curve a), and in 0.1M Na 2 SO 4 , after exposure NP-CPE to 0.510-3 mmol/L P-Aminophenol for 13 min, in a stirred solution (curve b). The reversible system could be observed at NP-CPE, with cathodic potential value, of -0.3 V and anodic potential value of -0.1 V.
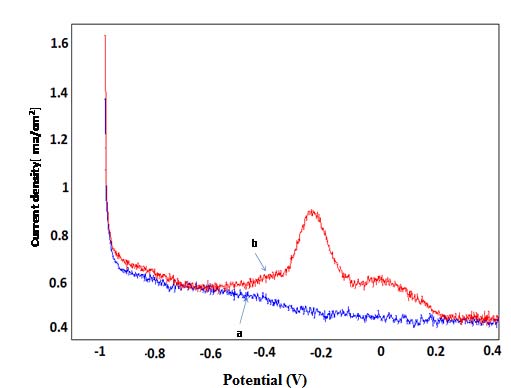
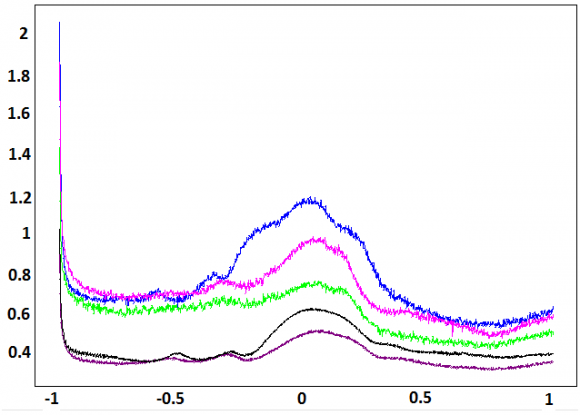
The square wave voltammetry (SWV) corresponding to the determination of P-Aminophenol, was recorded in the supporting electrolyte (curve a) and after, 13 min of accumulation in a solution containing P-Aminophenol (curve b).
The Square wave voltammograms are showed in Figures 4. A well-defined and enhanced peak is observed at NP-CPE, imprinted in P-Aminophenol solution. This peak is attributed to P-Aminophenol oxidation. The scheme 1 shows the proposed mechanism of this oxidation. Since its preferred habitat is dry sandy soil, it tolerates poor soils, such as those in coastal areas [31].
Firstly, the Moringa oleifera was prepared according the following procedure [30]. Moringa oleifera seeds collected for the analysis were shelled off and sun dried to maintain constant weight. The sun-dried seeds were grinded into powdered form using machine. The powdered was added to the solutions containing heavy metals. After 15 min of contact with moringa oleifera, the solutions were purified and analysis in electrochemical sensor.
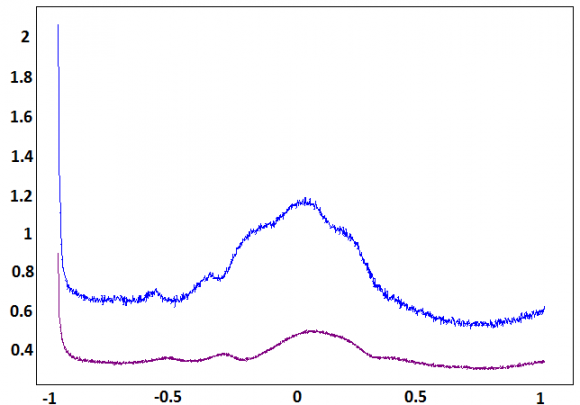
The SQWV's recorded at prepared electrode, in supporting solution containing p-aminophenol (curve a) and after addition of the moringa oleifera (curve b), are shown in Figure 7. The peak current decreased considerably after moringa treatment. This current density reduction is due to a sharp decline in paminophenol concentrations, which suggests that moringa has a strong complexing power of paminophenol (Figs. 7 and 8).We not that the solution pH was varied after moringa treatment. It was decreased from 7.2 to 6. Figure 7 and 8 shows the CV and SQW curves recorded, for different concentrations of moringa oleifera (MO), at NP-CPE was increased from 2 ml/200 ml (electrolytical solutions) to 13 ml/200 ml (electrolytical solution). Both the anodic and cathodic peak current increases linearly with the concentration of moringa oleifera and the plot of current versus concentration obeys Randles-Sevic equation, which implies that the electrode process is adsorption controlled reaction. It was also observed that the cathodic peak potential shift towards negative values and anodic peak potential shift towards positive side. This kind of shift in Ep in the cathodic and anodic. V en ml moringa The effect of the MO concentration of MO is investigated (Figure 9), this significantly affects the oxidation peak current of p-aminophenol. The peak current decrease greatly with MO concentration.
Similarly, the effectiveness of the Moringaenol to chelate p-aminophenol increaszs considerably with the MO concentration. (Fig. 10) In Figure 11, we present the SEM images, taken to the surface of the modified electrode; we can see that after MO treatment, the surface morphology changed dramatically with the advent of large compact crystals, leaving suggest that MO-p-aminophenol complex is adsorbed on the electrode by forming a continuous film.
9. IV. Conclusion
In conclusion, it was possible demonstrating the potentiality of the proposed electrode (NP-CPE) for determining P-aminophenol. Such a sensor is characterized by a higher sensitivity and reproducibility.
The Moringa oleifera seeds have the ability to retain p-aminophenol. The metal is sequestered by chemical sites naturally present in the moringa matrix. The chelating process is rapid and takes place under normal temperature and pressure. Moringa oleifera is an environmentally-friendly natural complexing most suitable for the treatment of water containing undesirable p-aminophenol concentrations. The removal efficiencies were 60 % for P-aminophenol. It is an eco-friendly technology that is economically more advantageous than other treatment alternatives.

![Figure 2 : Cyclic voltammograms recorded in electrolytic solution, at 100 mV/s, at a-carbon paste electrode, b-NP modified carbon paste electrode b) Electrochemical detection of studied metalsThe experimental conditions have been optimized and the response characteristics determined in a previous work[29]. The results obtained are:??p H ? ? 7 ??Pre conce ntra tion time = 13 min.In order to avoid the strong residual of reduction, the starting potential was fixed at -1V versus SCE. Fig.3shows a cyclic voltammograms performed between-1 V and 1 V for NP-CPE, in 0.1M Na2SO4 solution (curve a), and in 0.1M Na 2 SO 4 , after exposure NP-CPE to 0.510-3 mmol/L P-Aminophenol for 13 min, in a stirred solution (curve b). The reversible system](https://medicalresearchjournal.org/index.php/GJMR/article/download/1010/version/100577/2-Elecrochemical-Study-of-the-Capacity_html/9704/image-3.png)