1. Introduction
exually Transmitted Diseases (STD) refers to a group of infections and syndromes caused by pathogens, which can be transmitted or acquired through sexual activity (Workowski and Bolan, 2015). STDs represent one of the biggest health problems globally, especially in developing countries (Agaçdan and Kohl, 2006). In Brazil, some factors make it difficult to control this group of diseases, such as the scarcity of epidemiological data and high incidence associated with self-medication, causes the patients not to be informed and treated, favoring their dissemination (Araújo and Silveira, 2007). These infections can be divided into two groups, curable and non-curable, the latter being exemplified by Human Immunodeficiency Virus (HIV), Herpes virus, Hepatitis B, and Human Papillomavirus (HPV), all caused by viruses (Agaçdan & Kohl, 2006). HPV is the most common STD globally (Foldvari, 2011), being the most common to affect women (Burchell et al., 2006). According to the World Health Organization (WHO), about 291 million women are infected with HPV globally (Word Health Organization, 2018). Its transmission occurs through contact with the skin or mucous of infected individuals. However, its transmission does not necessarily occur by a fluid exchange. It can be transmitted via oral sex, manualgenital contact, or even to the baby by the mother during birth (Vento and PROADI-SUS, 2017). HPV is a non-cultivable, non-enveloped DNA virus (Ministério da saúde. Secretaria de vigilânciaemsaúde, 2010) belonging to the Family Papillomaviridae (Rosa et al., 2016), and to the genus Papillomavirus (de Lima Camara et al., 2003).
To date, approximately 250 types of Papillomavirus (Eça, 2004), its structure has a double DNA, with about 8000 base pairs (Nakagawa et al. 2010) (Paavonen, 2007), that is divided into three parts, L, E, and LCR. The genes found in the L (late) region are responsible for the formation of proteins that form the viral capsid. In the E (early) region, the genes are responsible for producing proteins involved in the malignancy of the host cell and proteins responsible for controlling the replication of the virus's DNA. The LCR (Late Control Region) is where promoters of these genes are located and the origin of DNA replication (Eça, 2004).
L1 is an important region, very conserved in all types of HPV, this region of he virus is used for genotyping (Nakagawa et al., 2010;Paavonen, 2007). The HPV subtypes differ in at least 10% in the nucleotide sequence of the L1 region (de Lima Camara et al., 2003). The types responsible for genital tract infection are divided into two groups. These groups are separated according to their low and high-risk oncogenic potential. The rst group is related to benign infection of the genital tract (Ministério da saúde. Secretaria de vigilânciaemsaúde, 2010). Those in the second group are highly related to carcinomas (Herraez Hernandez et al., 2013), causing subclinical lesions that can progress to cancer (Word Health Organization, 2018). These cervical lesions are known as cervical intraepithelial neoplasia (CIN). These neoplasms may be grade I, which only indicates the presence of the virus, or grade II and III, which are already precursor lesions of cervical cancer (Cohen et al., 2019). Therefore, the HPV the etiologic agent is a major contributor to the development of benign neoplasms such as laryngeal papillomatosis and malignant neoplasms such as oral and cervical cancer (Araújo et al., 2014 ;Nakagawa et al., 2010). The word cancer is a name for a set of 100 malignant neoplasms, having in common disorderly cell growth (Wild, 2014). Cervical cancer was one of the most common cancers affecting women worldwide (Bray et al., 2018) and the second most common type of cancer to hit women in Latin America (Wild, 2014) with high mortality in developing countries, second only to breast cancer (Bray et al., 2018). In 2018, 16,370 new cases of cervical cancer were estimated in Brazil, according to the National Cancer Institute -INCA. The most important thing for the success of the treatment is the discovery of the lesions in the early stages (Silva, 2019). With previous and periodic exams, it is possible to identify symptomatic and asymptomatic patients, thus controlling the spread of HPV (Bezerra et al., 2005).
The most common manifestations of HPV are genital warts. In these cases, the The most common subtypes are 6 and 11 (Borsatto et al., 2011). 70% of cervical cancer cases worldwide are associated with subtypes 16 and 18 (World Health Organization, 2008). Subtype 16 was the most common subtype in cases of cervical cancer in Brazilian regions (Rosa et al., 2009). The vaccine is the most suitable method to prevent infection, and currently, there are two types of prophylactic vaccines, intending to combat the spread of HPV and the lesions caused by the virus, the bivalent (subtypes 16 and 18), and the tetravalent (subtypes 6, 11, 16 and 18). These vaccines are indicated for women between 9 and 26 years (Silva et al., 2009).
Currently, HPV diagnosis is made using molecular biology tests. If lesions are identified during the prevention examination, a biopsy should be performed to distinguish benign from malignant (Brasil, 2010). Among the methodologies utilized for HPV diagnosis, the Flow Chip methodology stands out as it's a fast and sensitive molecular test for HPV detection and genotyping. DNA extraction is unnecessary when the samples are crude cells, such as cervical swabs, paraffin tissue, or liquid cytology (Herraez-Hernandez et al., 2013).
Therefore, this study aimed to determine the presence of the human papillomavirus and its high and low-risk subtypes in women who took the exam of genotyping HPV employing the Flow chip methodology, with the hypothesis that the presence of HPV is agerelated in the patients, so that the highest occurrence of the virus is in the age group of 16 to 25 years, in which sexual activity begins.
2. II.
3. Methods a) Sample collection
The samples of cervical-vaginal secretion were collected during the preventive exam, using liquid-based cytology from August 2018 to August 2019. The patients who took the exam of genotyping HPV at Cremasco Medicina Diagnítico, are residents of the cities of Vila Velha, Vitória, Cariacica, Viana, Guarapari, Serra and Domingos Martins. The samples are stored refrigerated (2 to 8 ° C) until the analysis, which was carried out at the Cremasco Medicina Diagnóstica.
4. b) Sample preparation
After the cells were seated on the bottom of the vessel, 200 µl of the suspension was removed and transferred to a 1.5 ml Eppendorf. The sample was then centrifuged at 2000 rpm for 3 minutes. The cell suspension was removed, leaving only an accumulation of cells (pellet) in the microtube. That pellet was resuspended in 400 µl of PBS 1X and again centrifuged at 2000 rpm for 3 minutes. The suspension was removed, leaving only about 30 µl of that. The polymerase chain reaction (PCR) was carried out with 4 µl of the suspension and an additional 36 µl of a mixture of HPV Primers, Enzyme DNA polymerase Hot Start and Enzyme Uracil DNA Glicolase. PCR is an in vitro technique formed by cycles typically consisting of 3 stages, denaturation, annealing and extension, amplifying DNA exponentially, generating billions of copies of a target sequence (Pereira, 2018). The target region for amplification is the L1 region during the PCR reaction. The reaction is formed by 53 cycles, the first at 25 °C for 10 min, the second at 94 °C for 3 min, and then a set of 15 cycles. 94 °C for 30 s, 42 °C for 30 s, 72 °C for 30 s, followed by 35 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s, followed by a 72 °C cycle for 5 min (Herraez-Hernandez et al., 2013).
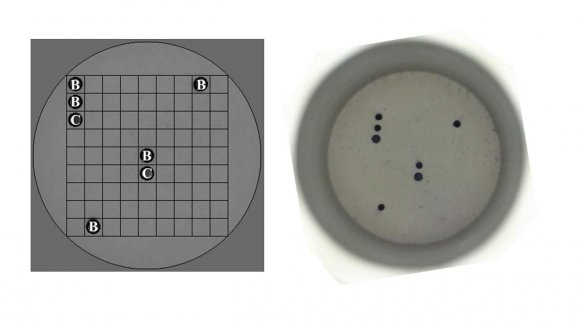
5. c) Flow Chip HPV detection
The Mobius® Multiplex HPV Kit was used for the qualitative detection and genotyping of 36 different types of Human Papilloma Virus, these being 16,18,26,31,33,35,39,45,51,52,53,56,58,59, 66, 68, 73, and 82 high oncogenic risk and the subtypes 6, 11, 40, 42, 43, 44, 54, 55, 61, 62, 67, 69, 70, 71, 72, 81, 84, and 89, low risk (Figure 1). The HPV ow chip protocol is based on the amplification of the HPV target region (PCR) and the application of these denatured products on the membranes of individual chips that contain probes for controls and universal HPV infection and the specic detection of 36 genotypes. After the reaction, the PCR product undergoes a denaturation of 95 ° C for 10 minutes in the thermal cycler. After the denaturation, the reverse hybridization process begins. Its specific DNA probes are immobilized on a chip composed of a nylon membrane. The PCR product binds to the probes present on the chip, and a colourimetric reaction generates the hybridization signal. This reaction produces a purple precipitate in the position that corresponds to the amplified fragment of PCR hybridized with the specific probe. This signal is read
6. Results
One hundred thirteen samples were analyzed, and 39 samples (26,5%) presented the HPV virus was distributed in high and low-risk subtypes (Table I), while 74 were not detected. The subtypes and their degree of risk of progressing to cancer were also identified among the positive samples. Patients with more than one HPV subtype (8) were identified, presenting low-risk and high-risk subtypes. The prevalence detected in this study was 34.5%.
7. F
The origin of the patients who underwent the examination was predominant in Cariacica (n = 55), followed by Vila Velha (n = 24). Other cities were Vitória (n = 8), Serra (n = 8), Domingos Martins (n = 5), Viana (n = 4), Guarapari (n = 1) and seven patients did not provide their address. The median age of HPV positive patients are 29. 25 to 75% of the patients are between 23 and 46 years old. For negative patients, the median was 36.5, and 25 to 75% of patients were between 32 and 48 years old. According to the test result -G (Williams), there was no significant difference between classes. However, with the patient index (D) divided by the total N of class (N) (Table II), it was possible to observe two peaks of infection, one occurring in class 16 -25 and the other in class 46 -55, being exactly in classes where the observed value was higher than the expected value.
IV.
8. Discussion
The prevalence of the current study was 34.5%, a similar result found by Holanda Jr et al. ( 2006) in Ceará, with a prevalence equal to 33.9% and lower than that found by Carestiato et al. (2010) in Rio de Janeiro, with a prevalence of 54, 3%. In the study by Araújo et al. (2014) in Belém, the prevalence was 24.1%, and de Sanjosé et al. ( 2007) identied a prevalence of 12.3% in South America. To better understand the occurrence of HPV in the population, women were divided into classes according to their age, and it was possible to observe that there was no association between infection and the patient's age. Araújo et al (2014) also found no association for both variables. However, through meta analyses, de Sanjosé et al. (2007) observed that the infection is more common in women under 25 years old. It is still unclear how age can influence the prevalence of HPV, but several studies show that the prevalence is higher in women under 25, with a decrease after that age (De Sanjosé et al., 2007;Matos et al., 2003). Adolescents are a the population of high vulnerability to sexually transmitted diseases as sexual intercourse begins, as they do not always use methods to prevent these diseases (34). The prevalence of HPV appears to have a bimodal behavior. It is possible to identify two peaks in the sample. The first in class is 16 -25 years old and the second in class, 46 -55 years old. De Sanjosé et al. (2007) observed the same behavior, who found an estimate of the prevalence of Higher HPV in women under 34. In the next group, there was a drop, and it increased again in the group of 45 -55 years old. This behavior was observed in women from almost all regions of the world. Giuliano et al. (2005) found the same situation on the border between Mexico and the United States of America. In Brazil, this behavior was observed by Pinto et al. (2011) in Eastern Amazon and by Rama et al. (2008) in the cities of Campinas and São Paulo. The HPV subtypes found in this study were 6,11,31,33,35,39,42,43,44,45,51,54,55,56,62,73, 81, and 84, of which eight subtypes are considered high risk and 9 low-risk (Herraez-Hernandez et al., 2013). The HPV tetravalent vaccine prevents the subtypes most associated with cervical cancer and genital warts.
In addition to identifying the virus in the sample, the genotyping carried out in this study is significant, allowing us to know whether HPV is the oncogenic type. When there is the persistence of an infection with a specific subtype of high-risk HPV, it can be decisive for the development of cervical neoplasms, interfering in the treatment of lesions. More intense approaches are indicated for treating infections caused by high-risk subtypes than low-risk ones (Burd, 2003). In most cases, the body can get HPV, but there are exceptions. With weakened immunity, the body cannot eliminate the virus (Ministério da saúde. Secretaria de vigilânciaemsaúde, 2010).
With the diagnosis of CIN I, the most indicated treatment is periodic exams to monitor the progression of the lesion, as only 11% of CIN I progress to CIN II or III. In the case of those progressing to high-grade injuries, a more intense the approach is needed than in the case of CIN I, and the most common is to perform an excision of the transformation zone (Derchain et al., 2005). One of the main cause of cervical cancer are persistent HPV infection, which is also associated with prolonged use of oral contraceptives, immunosuppression, and smoking. 6.385 deaths due to cervical cancer were registered in Brazil in 2017 (Silva, 2019). The treatment of precursor lesions is of great importance to reduce the incidence and mortality from cervical cancer (Burd, 2003).
V.
9. Conclusion
In conclusion, it was possible to determine the presence of human Papillomavirus in the analyzed samples, in which both high and low-risk groups were found. No association was found between the patient's age and the presence of HPV, so it is impossible to relate the patient's age to the infection, but this result may be related to the sample size. A study with a larger sample size would be necessary to investigate these variables more effectively.
| Results | N | Risk Degree | Subtype |
| Not detectable | 74 | - | - |
| Detectable | 39 | ||
| 3 | Undefined | *Genotype not identified | |
| 14 | Low risk | 6, 11, 42, 43, 44, 54, 62 and 81, 84 | |
| 14 | High risk | 31, 33, 35, 51, 56, 58, 73 | |
| 8 | Both | 6, 31, 39, 42, 43, 44, 45, 51, 55, 62 | |
| and 81, 73 |