1. Introduction
icks can be found on many hosts, including cattle, buffalo, horses, donkeys, goats, sheep, deer, pigs, dogs, and wild animals. Ticks are one of the leading monetary menaces to the cattle industry worldwide, affecting productivity, health and welfare. They are obligate blood-feeding ectoparasites that infest 80 percent of the cattle worldwide (Grisi et al., 2014). Livestock are the major source of livelihood but due to unhygienic in a herd and open grazing the chances of ectoparasite in livestock will be more common and causing heavy blood losses, irritation, hide damage and weight losses resulting in lower productivity (Kaur et al., 2016). Loss of appetite in heavily tick-infested cattle was found responsible for 65 % of the bodyweight reduction (Seebeck, 1971). These ectoparasites are among the most critical health problems like babesiosis, theileriosis, anaplasmosis and anemia . Ticks are highly responsible for economic losses worldwide, putting food safety at risk (Fernanedz-salas et al., 2017). In India, almost all the livestock species suffer from tick infestations India alone the cost of ticks and ticks born diseases (TTBDs) in animals has been estimated direct loss of more than 2000 crore per annum (Ghosh et al., 2007). According to the FAO (2004), 80 % of the world`s cattle population is exposed to ticks infestation and has estimated the impact of 7.3 US S/head/year. In addition to directly affecting their hosts, ticks are also the most important group of parasitic arthropods as vectors of pathogens that affect domestic animals and wildlife (Perez de Leon et al., 2020). Tick-borne pathogens are the foremost reason for transboundary livestock diseases, listed as notifiable by the World Organization for animal health (Esteve-Gasent et al., 2020). The TTBDs have been recognized as a major cause of production loss predominantly in tropical and subtropical countries of the world (De Castro, 1997;Parthiban et al., 2010;Lurthu et al., 2012;Arunkumar and Nagarajan, 2013;Mondal et al., 2013). Since the beginning of 20 th centuary investigators have documented numerous potential tick bio-control agents including pathogens, parasitoids and predictors of ticks (Samish & Alexseev, 2001). Entomopathogenic nematodes (EPNs) are parasites of insects. These are characterized by carrying specific symbiotic bacteria of the genus Xenorhabdus or Photorhabdus in their intestine (Boemare et al. 1993). Symbiotic bacteria play an important role in the pathogenicity of the nematodes bacteria complex to insect host and the subsequent reproduction of the nematodes in the host (Akhurst and Boemare 1990). EPNs are currently used as biopesticides to control several important insect pests worldwide (Shapiro Ilan et al., 2002).
EPNs are associated with symbiotic bacteria therefore they are extraordinary lethal to many important G soil insect pests. Biological control of insect pests using EPNs has gained importance in current years. Because they are highly virulent and killing their host within 24 to 48 hrs. They can be cultured easily in vivo as well as in vitro (on artificial diet), longer storage ability, have a high reproductive potential, broad host range, and can easily be applied in soil and foliage without adverse effects on non-target organisms (Georgis et al., 1991). They are safe for plant and animal health. Recently, it has been demonstrated that the entomopathogenic nematode, Steinernema carpocapsae has the potential to use as a biological control agent against cattle tick, Rhipicephalus microplus and Hyalomma savignyi, which is considered to be the most important tick parasite of livestock in the world (Monteiro et al., 2010). The major objective of the present investigation was to determine the effects of Steinernema carpocapsae on mortality of R. microplus and H. Savignyi at different levels of inoculums under laboratory conditions for effective bio-control of cattle ticks.
2. II.
3. Materials and Methods
The bio-efficacy test of indigenous EPNs strains of Steinernema carpocapsae STSLU and S. carpocapsae STUDR were conducted on important cattle tick, Rhipicephalus microplus and Hyalomma savignyi under laboratory conditions. Total sterilized 24 Petri plats were used for this experiment. The 25 cattle ticks were placed on Whatman filter paper no. 1 in each Petri plate and inoculated infective juveniles (IJs) from both the strains of S. carpocapsae at different inoculum levels viz., 50, 100, 150, 200 and 250 IJs/ Petri plate. All the treatments were replicated four times and placed at 20º C under B.O.D. incubator condition. The observations were taken on per cent mortality of cattle ticks after every day up to 5 days from the time of inoculation.
4. III.
5. Results
The experiment was conducted for evaluating the potential of the entomopathogenic nematodes (EPNs) indigenous strains S. carpocapsae against cattle ticks at different inoculum levels under laboratory conditions. The bio-efficacy was tested based on percent mortality of the cattle ticks R. microplus and H. savignyi were found susceptible against both the strains of S. carpocapsae STUDP-1 and STSLU under laboratory conditions. The maximum mortality of R. microplus was recorded 100 per cent with S. Carpocapsae STSLU followed by 97.5 with S. carpocapsae STUDP-1 @ 250 IJs per tick after 120 hrs (Table 1). Whereas the maximum per cent mortality of H. Savignyi was 97.5 per cent with S. Carpocapsae STSLU followed by 92.5 with S. carpocapsae STUDP-1 @ 250 IJs per tick after 120 hrs (Table 2).
IV.
6. Discussion
Tick mortality caused by EPNs seems to be due to the rapid proliferation of the nematode symbiotic bacteria within the ticks, since the nematodes do not go through their natural cycle within ticks and most infective juveniles die shortly after entry (Hassanain et al. 1999).
In vitro experiments demonstrated that tick hemolymph hinders the growth of EPNs (Zangi, 2003). Similar studies in this regard were made by who also reported that infective juveniles (IJs) of different EPNs strains (Steinernema glaseri, S. riobravus, S. carpocapsae, S. feltiae and Heterorhabiditis bacteriophora) appeared to be the most effective in killing ticks and invaded and killed 30 to 100% of replete females. Samish et al. (2000) reported that the mortality of Rhipicephalus bursa, and Rhipicephalus sanguineus adult ticks were recorded after 0.3 to 8.0 days of their exposure in Petri dishes to 5 entomopathogenic nematode strains. Maru et al. (2011) also recorded a cent per cent mortality of R. microplus was observed at 500 S. carpocapsae IJs/Petri plate after the fourth day of inoculation. Similar studies were made by Samish et al.
(1999) that the Mexican strain of Steinernema carpocapsae was most efficient, inducing 100% tick mortality at a concentration of 50 nematodes per square centimeter to our study 97.5 % mortality of ticks through EPN.
V.
7. Conclusion
The development of anti-tick biological control agents is still in its babyhood. Furthermore, the various steps required for commercialization of these products (production, storage and delivery) and education of consumers (storage, application and evaluation of results) are still in the future. Ticks infestation is a significant cause of economic losses to the dairy industry all over the world. At present, acaricides are mostly used for tick's control. To the extent possible, dairy farmers and veterinarians should make use of an integrated tick control strategy based on the utilization of biological control methods, breeding for tick resistance breeds etc. Nematodes are potentially used tools for ticks control because engorged ticks are susceptible to EPNs. However, the use of nematodes may be limited to defined ecological niches because their pathogenicity is reduced by low humidity or temperature and differences in the susceptibility among the various tick stage and species. Ticks have numerous natural enemies but Entomopathogenic have only a limited pragmatic role in tick's control. At present TTBDs control is mainly affected by the widespread use of acaricides like organophosphates, carbamates, pyrethroids, BHC/cyclodines, amidines, macrocyclic lactones and benzoyl phenyl ureas leading to various problems such as resistance, residues, environment pollution and high cost. These factors reinforce the need for alternative approaches to control ticks infestations. Several plants and herbs have been shown to possess anti-tick insecticidal, growth-inhibiting, antimolting and repellent activities. A number of reports are available on the use of vaccines for tick control on the horizon effect of different extracts of plant material on tick species. Due to severe problems associated with the continuous use of acaricides on animals, integrated ticks management is recommended. Increasing public health concern over tick-born diseases demands the strategic control of ticks on animals that transmit diseases to human beings. The development of improved formulations is also important. Finally, in-depth studies are needed to elucidate the interaction between nematodes and ticks under field conditions.
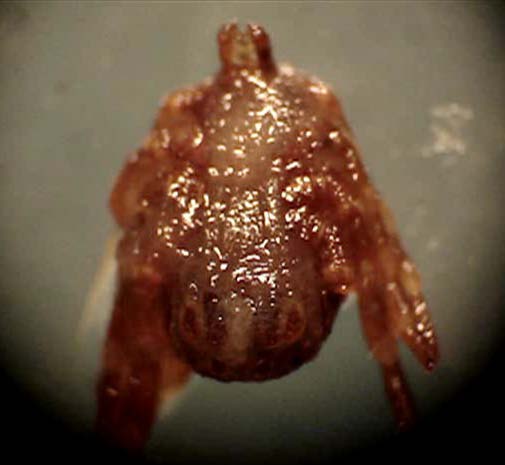
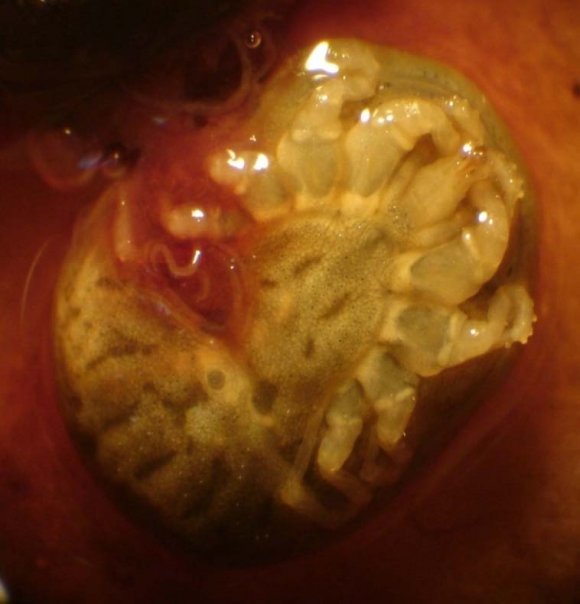
| microplus (Acari:Ixodidae). Parasitology Research. | entomopathogenic nematodes, Kimron Veterinary | ||||||
| 106: 821-826. | Institute, Bet Dagan, Israel, Journal of Parasitology, | ||||||
| 19. Parthiban M, Saranya R, Mahesh M & Raman M | 86 (4): 679-84. | ||||||
| (2010). Detection of parasite in cattle of Tamil Nadu | 23. Samish M, Gindin G, Alekseev E & Glazer I(2001). | ||||||
| using nested PCR. Tamil Nadu, J Vet AnimSci, 6: | Pathogenicity of entomopathogenic fungi to | ||||||
| 162-165. | different developmental stages of Rhipicephalus | ||||||
| 20. Pérez de León AA, Mitchell RD, Miller RJ, Lohmeyer | sanguineus, J Parasitol, 87: 1355-1359. | ||||||
| KH (2021). Advances in integrated tick management | 24. Seebeck RM, Springell PH & O'Kelly JC(1971). | ||||||
| research for area-wide mitigation of tick-borne | Alterations in the host metabolism by the specific | ||||||
| disease burden. In Book: Area-Wide Integrated Pest | and anorectic effects of the cattle tick | ||||||
| Management: Development and Field Application, | (Boophilusmicroplus) in food intake and body | ||||||
| Pereira R, Vreysen MJB, Eds, CRC Press: Boca | weight growth, Aust J Biol Sci, 24: 373-380. | ||||||
| Raton, FL, USA, p 251-274. | 25. Shapiro Ilan, Gouge DH & Koppeenhfer AM(2002). | ||||||
| 21. Samish M, Alekseev E & Glazer I (1999). Efficacy of | Factors affecting commercial success: case study | ||||||
| entomopathogenic nematode strains against | in cotton, turf and citrus. In Book: Gaugler R. (Ed.) | ||||||
| engorged Boophilus annulatus females (Acari: | Entomopathogenic Nematology. CABI, New York, p. | ||||||
| Ixodidae) under simulated field conditions. Kimron | 333-356. | ||||||
| Veterinary Institute, Bet-Dagan, Israel, Journal Med | 26. Zangi G (2003). Tick Control by Means of | ||||||
| Entomol, 36 (6): 727-732. | Entomopathogenic Nematodes and Fungi, In MSc | ||||||
| 22. Samish M, Alekseev E & Glazer I(2000). Mortality | thesis, The Hebrew University of Jerusalem, Israel. | ||||||
| rate of adult ticks due to infection by | |||||||
| No. of IJs/ insect | EPNs | 24 | Percent mortality at different time intervals (hrs.) 48 78 96 | 120 | |||
| 50 | S.carpocapsae STUDP-1 | 10.0 | 25.0 | 37.5 | 60.0 | 72.5 | |
| (18.44) | (30.00) | (37.76) | (50.77) | (58.37) | |||
| S.carpocapsae STSLU | 12.5 | 27.5 | 47.5 | 65.0 | 75.0 | ||
| (20.70) | (31.63) | (43.57) | (53.73) | (60.00) | |||
| 100 | S.carpocapsae STUDP-1 | 22.5 | 40.0 | 52.5 | 70.0 | 85.0 | |
| (28.32) | (39.23) | (46.43) | (56.79) | (67.21) | |||
| S.carpocapsae STSLU | 25.0 | 45.0 | 67.5 | 75.0 | 85.0 | ||
| (30.00) | (42.10) | (55.24) | (60.00) | (67.21) | |||
| 150 | S.carpocapsae STUDP-1 | 35.0 | 50.0 | 67.5 | 82.5 | 92.5 | |
| (36.27) | (45.00) | (55.24) | (65.27) | (74.11) | |||
| S.carpocapsae STSLU | 42.5 | 55.0 | 75.0 | 85.0 | 92.5 | ||
| (40.69) | (47.87) | (60.00) | (67.21) | (74.11) | |||
| 200 | S.carpocapsae STUDP-1 | 52.5 | 65.0 | 75.0 | 92.5 | 95.0 | |
| (46.43) | (53.73) | (60.00) | (74.11) | (77.08) | |||
| S.carpocapsae STSLU | 55.0 | 75.0 | 85.0 | 92.5 | 97.5 | ||
| (47.87) | (60.00) | (67.21) | (74.11) | (80.90) | |||
| 250 | S.carpocapsae STUDP-1 | 67.5 | 77.5 | 85.0 | 95.0 | 97.5 | |
| (55.24) | (61.68) | (67.21) | (77.08) | (80.90) | |||
| S.carpocapsae STSLU | 65.0 | 82.5 | 90.0 | 97.5 | 100.0 | ||
| (53.73) | (65.27) | (71.56) | (80.90) | (90.00) | |||
| Control | Water | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| SEm± | 0.637 | 1.302 | 2.709 | 2.806 | 2.443 | ||
| CD (0.05%) | 1.920 | 3.924 | 8.166 | 8.457 | 7.363 | ||
| CV (%) | 16.98 | 9.41 | 10.53 | 8.44 | 6.37 | ||
| No. of IJs/ insect | EPNs | Percent mortality at different time intervals (hrs.) 24 48 78 96 | 120 | ||||
| 50 | S. carpocapsae STUDP-1 | 5.0 | 12.5 | 17.5 | 32.5 | 57.5 | |
| (4.05) | (20.70) | (24.73) | (34.76) | (49.31) | |||
| S. carpocapsae STSLU | 5.0 | 12.5 | 27.5 | 47.5 | 67.5 | ||
| (4.05) | (20.70) | (31.63) | (43.57) | (55.24) | |||
| 100 | S. carpocapsae STUDP-1 | 12.5 | 25.0 | 32.5 | 52.5 | 70.0 | |
| (20.70) | (30.00) | (34.76) | (46.43) | (56.79) | |||
| S. carpocapsae STSLU | 15.0 | 25.0 | 47.5 | 65.0 | 75.0 | ||
| (22.79) | (30.00) | (43.57) | (53.73) | (60.00) | |||
| 150 | S. carpocapsae STUDP-1 | 25.0 | 42.5 | 55.0 | 67.5 | 80.0 | |
| (30.00) | (40.69) | (47.87) | (55.24) | (63.44) | |||
| S. carpocapsae STSLU | 30.0 | 47.5 | 57.5 | 75.0 | 85.0 | ||
| (33.21) | (43.57) | (49.31) | (60.00) | (67.21) | |||
| 200 | S. carpocapsae STUDP-1 | 37.5 | 55.0 | 65.0 | 80.0 | 87.5 | |
| (37.76) | (47.87) | (53.73) | (63.44) | (69.30) | |||
| S. carpocapsae STSLU | 42.5 | 65.0 | 75.0 | 85.0 | 92.5 | ||
| (40.69) | (53.73) | (60.00) | (67.21) | (74.11) | |||
| 250 | S. carpocapsae STUDP-1 | 45.0 | 62.5 | 77.5 | 90.0 | 92.5 | |
| (42.13) | (52.24) | (61.66) | (71.56) | (74.11) | |||
| S. carpocapsae STSLU | 57.5 | 72.5 | 82.5 | 90.0 | 97.5 | ||
| (49.31) | (58.37) | (65.27) | (71.56) | (80.90) | |||
| Control | Water | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| SEm± | 0.636 | 1.311 | 2.739 | 2.856 | 2.453 | ||
| CD (0.05%) | 1.909 | 3.933 | 8.217 | 8.567 | 7.359 | ||
| CV (%) | 16.87 | 9.29 | 10.57 | 8.47 | 6.36 | ||