1. Introduction
epsis is defined as life-threatening organ dysfunction resulting from a dysregulated host response to infection according to the Third Internal Consensus Definitions for Sepsis and Septic Shock of the Task Force 1 . Sepsis and septic shock are major health problems that affect millions of people worldwide each year 2 .
Regarding sepsis in the obstetric population, its incidence is different in developed and underdeveloped countries, varying from 0.96 to 7.04 per 1,000 women aged between 15 and 49 years. As for the estimated global mortality rates, they ranged from 0.01 to 28.46 per 100,000 women between 15 and 49 years of age 3,4 . Regarding national figures and based on the report made in 2021 by the National Institute of Health of Colombia, sepsis related to pregnancy corresponded to 3% 5 . According to the World Health Organization, sepsis is the third leading cause of maternal death in the world 6 . The main non-obstetric conditions associated with sepsis in pregnant women are urinary tract infections; however, in countries like Colombia, it is important to consider tropical infectious diseases such as malaria, which could be a pathology to take into account originating from sepsis 7 .
The pathophysiology of maternal sepsis is based on an excessive inflammatory response which includes extravasation of albumin and fluid, with subsequent intravascular hypovolemia. Likewise, the release of cytokines leads to a decrease in systemic vascularization, resistance and an increase in cardiac output 8 . During normal pregnancy, the human decidua contains high numbers of immune cells such as macrophages, natural killer (NK) cells, and regulatory T (Treg) cells. Consequently, the presence of immune cells at the implantation site is not associated with a "foreign body" response (the fetus), on the contrary, they have been attracted to facilitate and protect pregnancy 9 .
Regarding immunoglobulins, the immunoglobulin formulation enriched with IgM and IgA (12% IgM, 12% IgA and 76% IgG). Relevant mechanisms of action of IgM-and IgA-enriched immunoglobulins include opsonization and phagocytosis of causative pathogens 10 , neutralization of virulence factors, including bacterial endotoxins and exotoxins, as well as immunomodulation through interaction with complement factors and prevention of proinflammatory responses. Immunoglobulins have also been shown to down-regulate IL-2 production, resulting in significant inhibition of the proliferative response of human T lymphocytes in vitro, as well as in peripheral blood mononuclear cells stimulated with IL-2. lectin, In addition, in vitro and in vivo models have shown an increase in IL-10 after administration of IgM-and IgAenriched immunoglobulins. 12 .
Ideally, the indication of this medication is recommended up to 24 hours after the diagnosis of sepsis is made, however, it may be indicated when there has been no satisfactory evolution after 3 days of initial antibiotic therapy 13 . However, there is little evidence to support it.
Until now, only one published case report is known in Colombia in the context of an obstetric patient with a diagnosis of sepsis who was administered immunoglobulin G enriched with IgM and IgA with adequate clinical evolution, for which, taking into account the above, the objective of this report is to publicize and promote a new therapeutic option in obstetric patients with sepsis and inadequate response to conventional management.
2. II.
3. Case Reporte
We present a 20-year-old patient, primiparous, with a pregnancy of 16.3 weeks by ultrasound between weeks 11-14, without significant pathological or surgical history, obstetric history: first pregnancy, without any prenatal control, or paraclinical tests to date, never cytology had been performed, she did not remember the date of her last period, menarche at 13 years of age; history of immunization single dose of vaccine for COVID-19 (Astrazeneca 1 dose -did not remember the date), who was admitted to the emergency department of a low-complexity center (Hospital PASO La Manga) due to clinical symptoms of approximately 3 days of evolution, sudden onset, characterized by abdominal pain 10/10 according to the visual analog scale, located in the hypogastrium, radiating to the bilateral lumbar region and the right thigh associated with dysuria, bladder tenesmus and dizziness and nausea, without emetic episodes. Refers outpatient treatment with cephradine 500mg, 1 tablet orally every 8 hours for 2 days without improvement. On admission physical examination, vital signs were stable but febrile (blood pressure 100/60mmHg, heart rate 86bpm, respiratory rate 18rpm, ambient oxygen saturation 99%, temperature 38ºC); abdomen slightly painful on palpation in the hypogastrium and positive bilateral fist percussion, without vaginal leakage; It is managed in the emergency room with saline solution 500CC in bolus and continues at 80CC/hr, acetaminophen 2 tablets orally. Paraclinical tests were performed: normal blood cell count, urinalysis suggestive of urinary tract infection (bacteria ++, leukocytes 15xc, positive nitrites), negative acute phase reactants; Therefore, they consider that they have a urinary tract infection and initiate referral procedures to be managed and assessed by the gynecology and obstetrics service at Camino Universitario Distrital Adelita de Char.Upon admission to this center, vital signs were reported within normal parameters (blood pressure: 110/60 mmHg, heart rate 80 bpm, respiratory rate 18 rpm, oxygen saturation 98%, temperature 37ºC), on physical examination there were no signs of dehydration, abdominal pain in the hypogastrium of lesser intensity, without signs of peritoneal irritation, in the gynecological examination abundant non-fetid leukorrhea was evidenced, closed cervix and pain on mobilization of the cervix and on mobilization of the adnexa. A diagnosis of pyelonephritis associated with bacterial vaginosis was considered clinically and intravenous antibiotic management with cephalothin 1 gram IV every 6 hours was continued for 2 more days during said hospitalization. Subsequently, due to adequate clinical evolution and negative urine culture report at 48 hours (probably biased by antibiotic treatment previously received by the patient), she was discharged with cephradine 500mg, 1 tablet orally every 8 hours for urinary tract infection and metronidazole in ovules for the management of bacterial vaginosis, 1 ovule each day until completing 7 days with order of control urine culture 5 days after completion of antibiotic treatment.
Patient who is readmitted approximately 3 weeks later, with a pregnancy of 20.1 weeks, reporting the same symptoms of admission in the previous hospitalization, also comments that the outpatient treatment was not carried out adequately and the ordered control urine culture was not performed. He was admitted to the observation/emergency department with stable vital signs (blood pressure: 100/65 mmHg, heart rate 96 bpm, respiratory rate 18 bpm, oxygen saturation 98%, temperature 37.3ºC, normal fetocardia 140 bpm), without omSOFA criteria in that moment; Management was started with bolus saline solution and after maintenance, hyoscine ampule 20mg intravenously in a single dose, analgesic treatment with acetaminophen 1 gram orally and laboratories were requested again: complete blood cell count, partial urine count and acute phase reactants, HIV and serology for syphilis.Paraclinical tests were performed that same day, which were reported as follows (Table 2): HIV: human immunodeficiency virus, WBC: White blood cells, N: neutrophils, HB: hemoglobin, HTO: hematocrit, PLT: platelets. CRP: C-reactive protein.
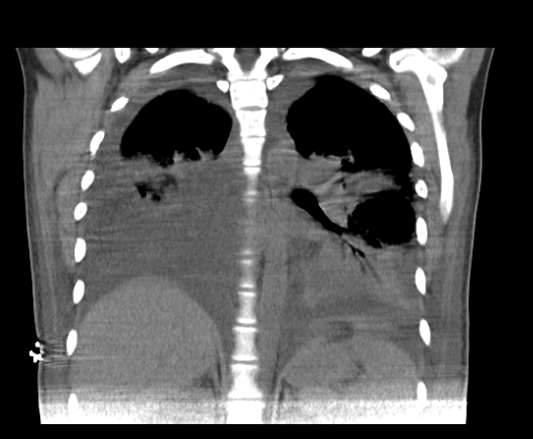
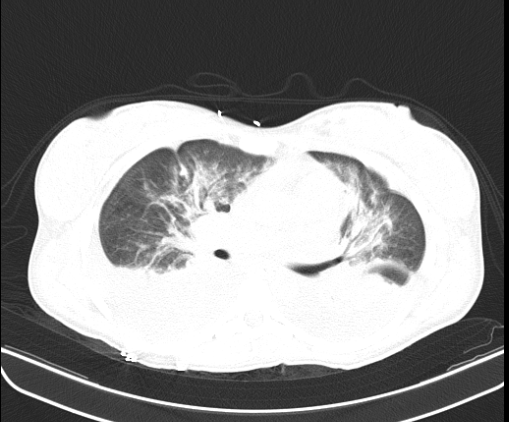
Pyelonephritis was again documented, for which the patient was hospitalized to start intravenous in-hospital antibiotic management (ampicillin/sulbactam, 3 g IV every 6 hours) and a urine culture was requested, renal and urinary tract ultrasound was performed, reporting a finding of bilateral hydronephrosis. grade II without findings of renal lithiasis or other alterations (figure 1). Patient who remained 4 days of hospitalization under antibiotic treatment mentioned above with stable evolution, however, on the 5th day of hospitalization he presented abrupt torpid evolution of his clinical picture with blood pressure figures with a tendency to hypotension, tachypnea, tachycardia (blood pressure 80/50mmHg, respiratory rate 28rpm, heart rate 112lpm, wakes up to verbal stimulation) (omSOFA: 2pts), then considering a diagnosis of sepsis of urinary origin, for which it was indicated to stagger antibiotic treatment to piperacillin/tazobactam at a dose of 4.5gr IV every 8 hours, a bolus of 2000cc (30cc/kg) was administered and basal fluids were continued at 100cc/hr; extension laboratories for sepsis were requested (Table 3) and transfer to the intensive care unit for comprehensive management was indicated. PT: prothrombin time, INR: international normalized ratio, PTT: partial thromboplastin time, CR: creatinine, LDH: lactic dehydrogenase, BUN: urea nitrogen, WBC: White blood cells, N: neutrophils, HB: hemoglobin, HTO: hematocrit, PLT: platelets. CRP: C-reactive protein, CL: chlorine, K: potassium, NA: sodium Based on platelet count findings, dengue with warning signs was considered as a differential diagnosis because it was located in an endemic area; but this diagnosis was later ruled out by both negative dengue IgG and IgM antibody tests. During her stay in the High Obstetric Risk Intensive Care Unit (seventh day of hospitalization and second day in the unit), she was assessed by the intensive medicine and critical care service, who, taking into account the intermittent fever, hypotension and systemic inflammatory response (tachycardia, tachypnea) despite management with broad-spectrum antibiotics (4 days of ampicillin/ sulbactam and two days of piperacillin/tazobactam at the doses described) and optimal fluid therapy, they consider a patient with septic shock and decide to start adjuvant therapy in the context of urinary focus shock with immunoglobulin G enriched with IgM and IgA at a dose of (5ml/kg/day) for 3 days. The patient's clinical evolution was monitored and she had persistent tachycardia (102 bpm) without tachypnea and without new febrile episodes. She was assessed by the infectious disease service (eighth day of hospitalization and third day in the unit) who considered continuing antibiotic escalation to ertapenem 1gr IV every 24 hours for 7 days due to persistent tachycardia and continuing with the last dose of immunoglobulin G enriched with IgM and IgA. The patient continued with satisfactory evolution, with blood pressure figures at goals, without requiring vasopressor support, with a progressive increase in platelet levels and improvement in the blood cell count (Table 4). On day 4 of intravenous antibiotic therapy with ertapenem (twelfth day of hospitalization, seventh day in the unit) the patient suddenly became tachypneic with saturations of 88%, for which a chest tomography was indicated (figure 2 and figure 3) where It showed a large left pleural effusion, which is why a thoracentesis was indicated, draining 620 cc of clear liquid without infectious characteristics in the bacterial culture cytology reading and a negative fungal test. Patient with immediate improvement after drainage of the pleural effusion; for which she was transferred to a general gynecological hospitalization receiving antibiotic treatment with stable vital signs, afebrile, without loss of fetal well-being evaluated by obstetric ultrasound, who completed antibiotic treatment and proposed adjuvant immunotherapy scheme with immunoglobulin G enriched with IgM and IgA with adequate drug tolerance and favorable clinical course.
No adverse events were recorded during the hospitalization of the mother and the administration of immunoglobulin G enriched with IgM and IgA, nor were subsequent maternal and perinatal complications documented during the course of pregnancy.
4. Discussion
Maternal sepsis corresponds to an obstetric emergency, and a leading cause of maternal and perinatal morbidity and mortality 14 . Timely and targeted antibiotic treatment and intravenous fluid resuscitation are essential for the survival of patients with suspected maternal sepsis. If the patient presents with septic shock or does not respond to initial treatment, multidisciplinary management and therapeutic alternatives are necessary.
Among these alternatives are immunoglobulins.
Specifying the management and treatment of this pathology, it is considered that it is generally similar in both the pregnant and non-pregnant population. The international guidelines for the management of sepsis and septic shock 2021 proposed by the campaign surviving sepsis 15 establish some key points about what should be done when the diagnosis of sepsis is certain; these points are specified in table 5: The guide for sepsis care in pregnancy of the Royal College of the United Kingdom, and the Australian guide for sepsis in pregnancy consider that IgG enriched with IgM and IgA can be indicated as an alternative therapy in septic shock secondary to staphylococci and streptococci, recommending its use. use, but little evidence of its usefulness in sepsis due to gram-negative microorganisms has been reported; however, more studies and literature are expected to support the use of immunoglobulins in the context of obstetric sepsis of any origin 16,17 .
Since 2012, the use of enriched immunoglobulins has been endorsed in the context of pregnant patients with sepsis who do not respond to initial management with antibiotics and intravenous fluids. According to the Sánchez-Padrón Guidelines for the care of severe sepsis in obstetric patients 18 , the currently recommended dose for the pregnant population is 5 ml/kg/day (250 mg/kg of body weight/day) for 3 consecutive days with an infusion rate of 0.4 ml/kg/h; Additional infusions may be required depending on the clinical course of each patient. However, it should be noted that this drug is not commonly prescribed, few institutions routinely use it for severe infections, and there are still no established guidelines for how and when to use it.
There are very few reports that support the use of this type of medication in pregnant patients. A case of a patient in Turkey with a 29-week pregnancy and sepsis secondary to resistant acinetobacter is described. After 7 days of starting antibiotic therapy, her condition worsened, so they decided to start enriched immunoglobulin G (20 mg/kg/hr initial dose and continue 10mg/kg/hr for 68hrs) with subsequent response to management with immunoglobulins without associated adverse effects 19 . In this case, even higher doses were used than those usually recommended in this type of population. Likewise, there is another case published in the United Kingdom 20 of a patient who had a preterm birth at 32 weeks of gestation, developed sepsis due to GBS (group B streptococcus) 12 hours postpartum and, in addition to antibiotic therapy, therapy with enriched immunoglobulin G (in said study no dose or duration is specified). Two days after the established treatment, it begins to stabilize, a fact that justifies that the earlier the medication is started, the better results in terms of clinical evolution are expected. In Colombia to date, there is only one reported case of a patient with a 36-week pregnancy, who presented septic shock secondary to a gastrointestinal infection and progressed to multisystem organ failure in whom adjuvant therapy with IgM-enriched immunoglobulin was started with a good outcome. response and no maternal-perinatal complications at a dose of 5ml/kg/day for 3 days 21 ; As administered in this case report, the patient in question had an excellent response to the established treatment, reducing the systemic inflammatory response in the context of septic shock of urinary origin.
IV.
5. Conclusion
A case of a 20-year-old primipregnant woman, previously healthy, with pregnancy in the second trimester, who developed sepsis of urinary origin, was presented. Initially, it was treated as a urinary tract infection with antibiotic management, but the patient did not respond to said therapy. Subsequently, antibiotic treatment was staggered and enriched immunoglobulin G was used as adjuvant therapy. Despite the initial form of presentation, the patient responded favorably from the clinical and paraclinical point of view after receiving the aforementioned treatment without presenting adverse effects or maternal-perinatal complications and continues her prenatal check-ups without documented sequelae. Among the strengths of this study, the use of a drug that can be used in pregnant women as adjuvant therapy in the context of sepsis and that could improve the maternal and perinatal prognosis should be highlighted. However, more studies and investigations should be carried out to evaluate the presence of adverse effects with the use of this drug in the short and long term, both in mothers and in newborns.
| omSOFA variables. | Points | 0 | 1 | 2 | ||
| PaO2/FiO2 respiration. | > 400 | 300-400 | < 300 | |||
| Platelets | ? 150.000/mm3 100.000 -150.000/mm3 | < 100.000/mm3 | ||||
| Bilirrubins (mg/dL) | ? 1.17 | 1.17 -1.87 | > 1.87 | |||
| Mean arterial pressure (MAP) | PAM ? 70 | < 70 | Need for vasopressor to maintain MAP | |||
| Consciousness state | Alert | Wake up to verbal encouragement | Arouses only to physical stimulation or pain | |||
| Serum creatinine(mg/dL) | < 1.0 | 1.0 -1.36 | > 1.36 | |||
| Sepsis in the pregnant population is a serious | ||||||
| entity that has both short-term and long-term maternal | ||||||
| and perinatal complications; such complications can | ||||||
| even lead to maternal or neonatal death. Important | ||||||
| neonatal complications include: preterm delivery, | ||||||
| neonatal sepsis, chronic lung disease, brain injury | ||||||
| secondary | to | maternal | infection, | and | ||
| neurodevelopmental disorders | ||||||
| PATIENT VALUES | REFERENCE VALUES |
| HIV: NEGATIVE. | HIV: NEGATIVE. |
| TREPONEMIC TEST: NON-REACTIVE | TREPONEMIC TEST: NON-REACTIVE |
| COMPLETE BLOOD COUNT: WBC: 13000, N: 78%, HB: 10, HTO: 33, PLT: 144.000. | COMPLETE BLOOD COUNT: WBC: 10.000-15.000, N: 60% -80%, HB: 12 -15, HTO: 38 -48, PLT: 150.000 -450.000 |
| CRP: 1.34 | CRP: 1 -3. |
| UROANALYSIS: NITRITOS NEGATIVOS, LEUCOCITOS INCONTABLES, BACTERIAS ++. | UROANALYSIS: NITRITOS: NEGATIVOS, LEUCOCITOS: 0-5XC, BACTERIAS ESCASAS. |
| TP: 13. INR: 1.02. TPT: 29, 8. ALT: 13, |
| 8. ASAT: 20, 9. |
| CR: 0,8. LDH. 196. BUN: 14. LACTATO: |
| 1,9. |
| HEMOGRAMA: WBC: 13000, N: 78%, |
| HB: 7.9, HTO: 24, PLT: 53.000 |
| PCR: 0.04 |
| CL: 105. K: 3,1. NA: 137. |
| WBC: 12.000 |
| N: 76% |
| HB: 11 -HTO: 34 |
| PLT: 180.000 |
| KEY POINTS |
| 1. Measure the lactate level. |
| 2. Obtain blood and urine cultures before |
| starting antibiotic therapy. |
| 3. Administer broad-spectrum antibiotics. |
| 4. Administer 30 cc/kg of crystalloids to |
| avoid hypotension. |