1. Introduction
ransdermal administration is acknowledged as a promising approach for local and systemic medication delivery. Transdermal medication delivery avoids hepatic first pass metabolism and enhances patient compliance. However, the stratum cornea's highly structured structure acts as a barrier to drug permeability and must be changed to give poorly penetrating medicines [1] . The use of chemical penetration enhancers would greatly increase the number of transdermal medication molecules.
In Saudi Arabia, where it affects about onequarter of the adult population, hypertension is increasingly becoming a problem. In 2007, the prevalence of hypertension in Saudi Arabia was 26.1%. Male cases predominated over female instances (28.6 vs 23.9, respectively). Hypertension was more prevalent (by roughly 27.9%) among urban residents than rural residents (occurrence of 22.4 percent). The risk of hypertension increases with age, even if significant and effective preventive actions have been taken [2]. Patients with hypertension need ongoing treatment. Sometimes a lifetime of treatment is necessary. The majority of hypertension medications prescribed are angiotensin II receptor blockers. Here are a few instances of the angiotensin II receptor blockers with high oral bioavailability: Nebivolol is 13%, candesartan is 15%, valsartan is 10%-35%, and olmesartan is 28.6%. [3]. A highly cardioselective b1-receptor blocker with a vasodilatory effect, nitric oxide mediates the action of nebivolol HCL (10 mg). White powder, NNdimethylformamide (DMF) is soluble in methanol, dimethylsulfoxide, and DMF but not in ethanol, propylene glycol, or polyethylene glycol. The half-life of the active isomer, d-nebivolol, is 12 hours. Nebivolol plasma concentrations peak 1.5-4 hours after administration. Drug binds to protein 98% of the period in vitro. There are no reports of transdermal nebivolol patches. The mechanical properties and medication release pattern of these patches will be examined in vitro. [4].
2. II.
3. Materials and Methods
4. a) Materials
Nebivolol HCL was purchased from Hyderabad's Hetero Drugs Ltd. We bought Tween 80 from Gattefosse in Mumbai. The reagents used were all of the analytical variety. SD Fine Chemical, based in Mumbai, India, supplied the soyalecithin, Span and Tween 80, Hydroxypropyl E 15 and HPMC E 5, and other products. Other substances and reagents were of analytical quality.
5. b) Methods
6. Preparation of Nebivolol HCL Loaded Transfersomes
The flask combination was then given ethanol and chloroform [5]. In the same flask, the drug loading was completed. Each element was dissolved by shaking the solution. The inner surface of the flask was coated with a thin dry lipid layer by a rotary evaporator. The solution was added to an 800 ml rotary vacuum flask evaporator, which was spun at 60 rpm for 30 minutes to create a thin coating on the flask walls.
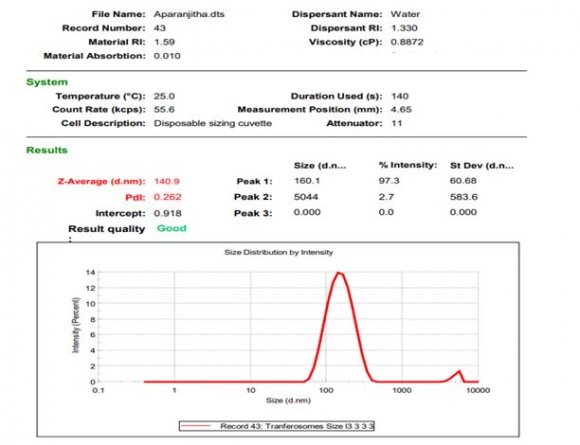
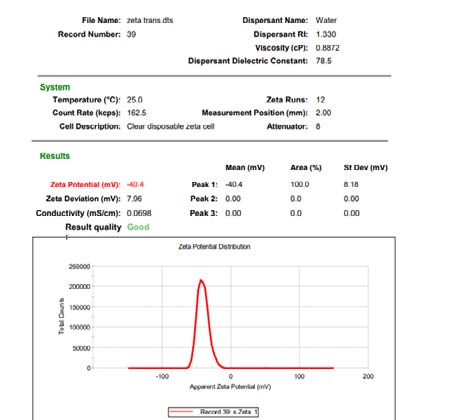
Preliminary formulations [6] Sizing, polydispersity index and zeta potential estimation The prepared transfersomes' vesicle size, PDI, and zeta potential were measured by light scattering using the Malvern Master seizer and water as dispersion.
7. Entrapment efficiency
After vesicle disruption, the drug concentration in trasferosomes was measured. Trasferosomes containing medication were centrifuged for 30 minutes at 14,000 rpm. The filtrate was analysed. [7].
8. Drug content determination
This was measured by dissolving 100 mg of the formulation in 10 mL of ethanol. The blend was tested.
9. Preparation of transdermal patch
As a backing membrane, aluminium foil was used to cast the transferosomal mixtures into transdermal patches. The optimal HPMC: PVA ratio (1:1) was chosen [8]. Plasticizer glycerol was added to the mix.
10. Characterization of Transfersomal patch
Visual inspection was performed on all of the transdermal patches to ensure that they were of proper colour, clarity, flexibility, and smoothness.
11. Uniformity of weight
The average weight of the individual batch was established by weighing five separate patches from the same batch. A considerable deviation from the average weight of five should not be observed in any individual weight [9] .
12. Moisture content
The film was weighed and then placed in a desiccator with calcium chloride for at least 24 hours to dry out the moisture. The film was weighed several times until it exhibited a consistent weight on the scale. The moisture content was calculated as the difference between the constant weight taken and the beginning weight, and it was expressed as a percentage (by weight) of the total weight of the sample.
13. Thickness
The thickness of the patch was measured with the use of Vernier callipers. The thickness of the patch was measured at three distinct locations on the patch, and an average was determined. It is critical to understand the uniformity of patch thickness since it is directly related to the precision of the dose delivered in each patch.
14. Folding endurance
The folding endurance of all of the formulated patches is measured manually by a third party. Using a patch (2 x 2 cm2), a strip was cut and folded at the same spot over and over again until it broke. The brittleness of a patch is determined by the number of times it can be folded in the same spot without breaking or cracking [10] .
15. In vitro dissolution studies
Permeation experiments were carried out in a Franz Diffusion cell of the vertical kind. It was decided to keep a semipermeable membrane on a diffusion cell with an effective diffusion area of 2.303 cm2 in order to test it. It was maintained at 37 0.5°C throughout the trials with a 22.5 ml phosphate buffer at pH 6.8 as the receptor fluid, which was agitated at 100 rpm in the receptor compartment [11] . The amount of drug that permeated was measured by graphing the cumulative amount of drug that permeated against the passage of time [10].
16. In vitro skin permeation study
Prior to usage on experiment day, the frozen hog skin samples were thawed and examined for nicks and holes. The skin samples were cut to the proper size and placed on a horizontal type Franz cell on an SFDC-6 transdermal diffusion cell instrument (Logan, USA) with the stratum corneum facing the donor compartment and the dermis facing the receiver cell. Nanotransfersome formulations were applied to the surface of the rats' skin in a non-occlusive way. This experiment used ethanolic phosphate buffer saline (epbs) as the receptor medium (pH 7.4, 20:80). A small magnetic bead moving at a speed of 100 per minute revolutions was used to stir the receiver cell vehicle, which was held at a temperature of 37 degrees Celsius. One millilitre of receiver medium had to be sampled, and an equivalent amount of fresh vehicle had to be added. The samples that were eliminated were assessed using the HPLC technique. A number of metrics were calculated, including the total amount of medication that was absorbed, the lag time (Tlag), the permeability coefficient (Kp), and the enhancement ratio (ER). [12,11] .
17. III.
18. Results and Discussion
19. a) Screening of edge activators
Span 80, Tween 80, and Labrosol were selected and subjected to additional screening based on the size of the vesicle and the effectiveness with which it was entrapped.
After preparing the nano vesicles using the above-mentioned procedure, they were assessed for their size and entrapment efficiency.
20. Table -1: Formulation of nebivolol hydrochloride transferosomes using various edge activators
No. 1 in the table The nanovesicles produced using labrosol (NT7, NT8, and NT9) have shown higher size and improved trapping efficiency as compared to earlier formulations, as shown in table No.1. According to this evidence, increasing the concentration of phospholipids increases the drug's ability to be captured; this is consistent with the facts. The edge activator Labrosol was found to be more suitable for the selected medicine's transfersomal formulation based on the results in Table 1. [13 ].
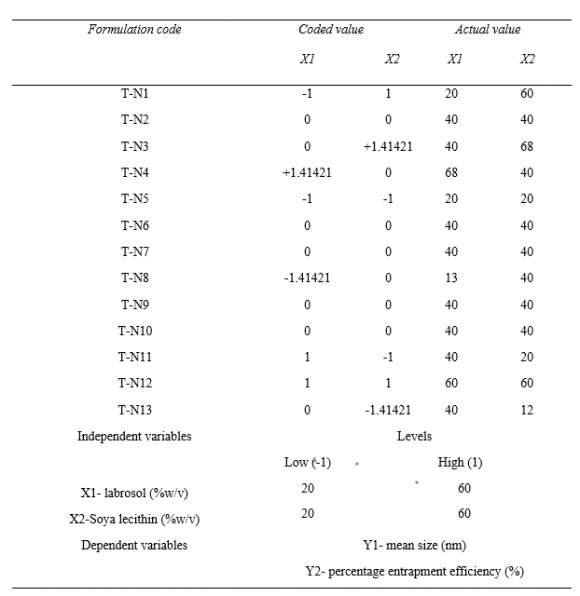
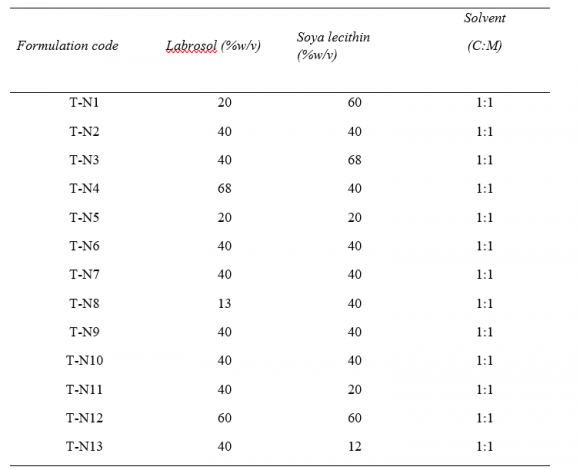
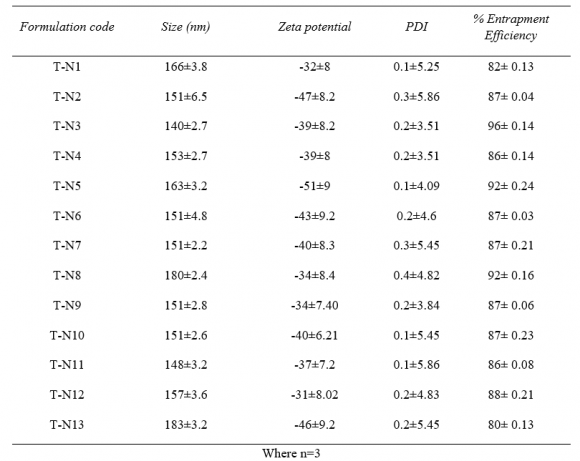
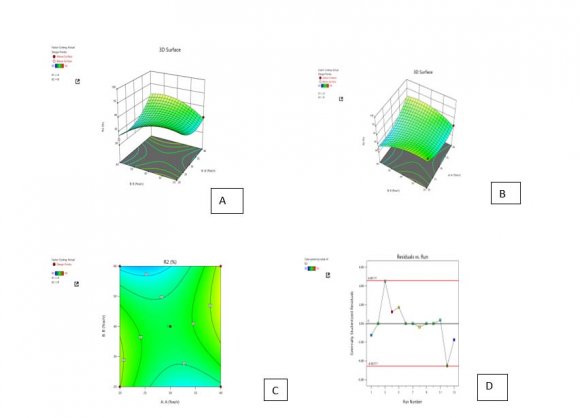
21. Optimization of the formulation by Central Composite Design
The central composite design having two independent variables (X1&X2) was used to study the effect on dependent variables (Y1&Y2). As per the design (table no.2) total 13 runs were generated out of which 9 with unique combination were observed. The formulations were prepared (table No.3) according to the generated combinations of the design and evaluated for the responses like %entrapment efficiency (Y1) and particle size (Y2) mentioned in table no.-5.9. The formulations have shown wide range in dependent variables, particle size having range of 140nm-183nm and %entrapment efficiency having 40-96% as shown in table no.
22. Preparation of transdermal patches
The transdermal patches were made with varied doses of HPMC E15 and E5. Water and methanol are utilised as the solvent, and polyethelenglycol (PEG)-400 is used as a plasticizer. Transdermal patches are first made, as indicated in Table-5. As can be seen in table no. 5, the created formulations underwent evaluations for weight variation, thickness, folding endurance, moisture absorption, and percentage drug release. The formulations were made using the solvent casting method, with the help of the plasticizer PEG-400 (%w/v), the polymers HPMC E 15 and 5, and water as the solvent. [15] .
23. Table-5:
24. Table-6: Evaluation of transdermal patch with nebivolol hydrochloride
The formulations made with pure medication have drug release in the range of 60-70% invitro across dialysis membrane, according to table No. 6. The folding endurance is inacceptable range for all the prepared formulations [16]. It was discovered that all of the created formulations maintained the proper weight, thickness, and diameter. The transdermal patches' moisture absorption capacity is optimal.
25. Preparation of transfersomal patches
The formulations with nanocarriers are made using an optimised nano formulation, in which the drug equivalent of 10 mg is added to a mixture of HPMCE15/HPMC E5, plasticizer (PEG-400), solvent (water), and other ingredients as given in table no. 5.18. Evaluation is done on the prepared formulations [17].
Table -7
26. Evaluation of transfersomal patches
The evaluation of transfersomal patches were carried out for Folding endurance, weight variation, thickness, moisture content and percentage drug percentage [18] .
27. Table-8: Evaluation of transdermal patch with nebivolol hydrochloride nanocarriers
The patches made with HPMC E5 have demonstrated better drug release (24hrs) as compared to other formulations, according to the examination of the transfersomal patches [19]. The invitro drug release has improved in the transfersomal patches with increases in HPMC E5 concentration. When comparing the drug release studies using transdermal patches with drugs and transfersomal patches, a comparative drug release bar graph like that in Fig. 4.13 is generated. It is clear that the inclusion of flexible nano vesicles that improve diffusion has an impact on the drug release from transfersomal patches [21,20].
28. In-vitro evaluation of transdermal patch with nanocarriers using pig skin
Pig skin was used to assess transfersomal patches for skin permeation experiments. Calculations were made for the flux, penetration coefficient, total amount of drug absorbed over the course of 24 hours, enhancement ratio, and lag time (hours) [22]. The results were analysed, and it can be seen from Table No. 9 that all the transfersomal formulations have better permeation properties. However, when compared to all the formulations, patches with HPMC E5 of 2.5% with plasticizer 1.5% and nano formulation have shown better permeation results, having flux of 43 1.2 (g/cm2/hr) and permeation coefficient of 14.5 (cm/hr) (Kpx103)-9, Enhancement Ratio-2.8, Lag time(hr)-0.2 and Q 24 (µg/cm 2 )-733± 1.33 [23,22].
29. Skin irritation study
The animals used in the investigation of cutaneous irritation were guinea pigs. Studies on skin irritation were conducted for 14 days, and the results were tabulated accordingly. The skin irritant caused irritation with both minor and definite erythema, and after 12 days, clearly discernible edoema was created. When compared to this, neither the placebo nor the optimised batch displayed any irritation until 11 days later [24].
30. Stability studies
In accordance with ICH recommendations, the stability studies were carried out using optimised patches under accelerated stability circumstances. Every 0, 15, 30, 60, and 180 days, the patches were examined for moisture absorption, folding endurance, drug homogeneity, and in vitro drug release through the skin [25]. IV.
31. Conclusion
The results of in vitro drug release demonstrate that produced patches released drugs more effectively than conventional transdermal patches. The skin permeation investigations of the improved transfersomal patch (HPMC E5 3% w/v, 1.5% w/v PEG-400, 2% v/v nano suspension, i.e. 40% w/v Labrosol with 68% w/v soy lecithin) revealed flux of 43 (g/cm2/hr), permeation coefficient (cm/hr) -14.5, and absorption rate (g/cm2/hr), Enhancement Ratio of 2.8, non-fickian diffusion in the Korsemeyer-Peppas model with n = 0.8, and zero order kinetics type of drug release are characteristics of the optimised patches. When compared to the placebo batch and the optimised batch, the optimised formulation has demonstrated better stability. Therefore, based on the results of the current investigation, it can be concluded that transfersomal patches have better drug release qualities than conventional transdermal matrix patches. A regulated drug release of the medication has also been demonstrated via the transdermal patches.