1. I. Introduction
iplozoid monogeneans are gill ectoparasites of freshwater, mainly cyprinid fish are represented by two dozens of species in Europe (Khotenovsky 1985). Systematics of the family remains problematic due to a relatively high interspecific similarity in morphological features and limited number of species included in molecular comparisons (Matejusova et al. 2001;2004;Gao et al. 2007;Civanova et al. 2013;Avenant-Oldewage et al. 2014). Therefore, supplementary approaches would help in the assessment of species delimitation and/or phylogeny. To date, only few studies have been focused on diplozoid cytotaxonomy and none from the Kashmir valley. Koroleva (1968a,b; showed chromosome morphology of six species from various fish hosts, and showed specified cytogenetic characteristics of four of them. Species of Paradiplozoon bliccae, Paradiplozoon sapae, Paradiplozoon nagibinae, Paradiplozoon pavlovskii and Paradiplozoon homoion has 14 acrocentric elements in their diploid set (2n=14), while Diplozoon paradoxum has 2n=8. Baer and Euzet (1961); Bovet (1967); Koroleva (1969) showed that three undetermined diplozoids showed either 14 or 10 chromosomes in diploid set and Koroleva (1968b) studied two other species Eudiplozoon nipponicum and Paradiplozoon megan, revealed n=7 on the basis of meiotic bivalents without any information on the chromosome morphology. The present study describes for the first time the chromosome structure and number of three Diplozoon spp. from Kashmir Valley. The three species of Diplozoon, parasitizing Schizothorax species, have been the objects of our cytogenetic study, aimed at a comparison of a structure of their chromosome sets and an analysis of hypothetic routes of karyotype evolution within the group.
The Clinostomidae Luhe, 1901 is a family of digeneans, the members of which live in the oral cavity, gills, gill covers, eye sockets, operculum, fins, and gill lamellae of fishes. Due to the high degree of morphological variability within the same species, Clinostomum has been subjected to several taxonomic revisions (Gustinelli et al., 2010). The application of a karyology in parallel to morphological study may be particularly important for the identification of Clinostomum species described in the past only on the basis of morphological features.
The purpose of this study is to find out the chromosome number of trematodes from different vertebrate hosts. Differentiate trematodes on the basis of the karyological characteristics and role of these studies in cytotaxonomy. Lastly to find out the general aspects such as trends of karyotypic evolution and sex mechanism of trematodes.
2. II. Materials and Methods
Whole living specimens were placed in physiological saline (0.65% NaCl) containing colchicine (0.05%) for 3-4 hours at room temperature then transferred into distilled water for about one hour for hypotony and fixed in ethanol-glacial acetic acid (3:1), with two changes, 15 minutes each. Spread preparation of mitotic and meiotic chromosomes was made as described by Petkeviciute and Leshko, 1991. Small posterior mature portions of fixed worms were transferred into drop of 60 % acetic acid on a slide and torn into fine pieces with the help of tungsten needles.
The slides were then placed on a heating plate at 45 0 C and the drop of cell suspension was slowly drawn along the slide until it evaporated. Slides were dehydrated in an ethanol series (30%, 50%, 70%, 90% and 100%, 5 minutes each) and stored at -20 0 C until use. Slides were stained with 4% Giesma solution (pH. 6.8) in phosphate buffer for 30 minutes, rinsed in tap water and allowed to dry. The best chromosome plates were photographed and used for morphological studies.
For karyotyping, chromosomes were cut out of the photomicrographs and paired on the basis of size and centromere position. Relative lengths of chromosomes were calculated by the division of the individual chromosome length by the total haploid length and centromeric indices (ci) were determined by division of the length, i.e;
3. Ci =
Length of short arm x 100
4. Total length of chromosome
Measurements are based on all chromosomes from 10 best metaphase spreads of parasites. The terminology relating to centromere position follows that of Levan et al., 1964. A chromosome is metacentric (m) if the ci falls in the range of 37.5-50.0, submetacentric (sm) if 25.0-37.5, subtelocentric (st) if 12.5-25.0 and acrocentric (a) if < 12.5. When the centromere position was on the borderline between two categories, both are listed.
5. III. Results and Discussion
6. a) Chromosomes of Trematodes
A number of workers on trematode cytology, especially Jones and his co-workers, have pointed out the possible taxonomic value of the study of the numbers, volume, and/or size and shape of chromosomes, and much of our information along these lines is based upon their investigations. Among the Monogenea only the Polystomidae have been investigated for the purpose of determining the chromosome numbers.
Polystoma integerrimum [Frolich, 1791] has the haploid number of 4 chromosomes and Gyrodactylus elegans has 6. This could point to a dalyelliid ancestry (n = 2 in all species studied) although more primitive ancestors of the dalyelliids may have had a larger basic number of chromosomes (Table 1). Among the Digenea, the paramphistomatids have been regarded as the most primitive group, but from a cytological standpoint the basic chromosome number is quite variable and is therefore of little guidance in determining possible relationships. For example, Gigantocotyle shows a basic number of 6, Gastrothylax and Zygocotyle have 7, Cotylophoron and Diplodiscus have 8, while Heronimus (chelydrae) has 10. No one family of the rhabdocoel group could be regarded as being directly ancestral based on such divergent records. As much as the exact phylogenetic relationships of the remaining families of the Digenea are not as yet fully determined, even on morphological grounds, and the various authorities disagree as to their proper taxonomic positions, no attempt will be made to discuss our knowledge of chromosome numbers in any significant succession. The diploid chromosome numbers vary among studied digenean taxa, from 12 to 28 (Bariene, 1993); chromosome sets with 20 or 22 elements predominate. But 56 chromosomes were found in diploid sets of Clonorchis sinensis [Cobbold, 1875] (Park et al., 2000). Allocreadiid species possess comparatively large chromosomes, up to 13-14 mm, but low haploid numbers of six, seven or eight were recorded in most species (for a review see Petkeviciute & Staneviciute, 2008). The chromosome complement of Cercariaeum crassum [Wesenberg-Lund, 1934] is unusual among digeneans due to the low number, 2n=10. The karyotype is composed of large and exclusively biarmed chromosomes. Such a karyotype presumably results from a decrease in chromosome number through centromere-centromere Robertsonian fusions that have affected mono-armed chromosomes leading to the formation of large metacentric elements. Comparative analysis of chromosomes of related trematode species indicated that the reduction of chromosome numbers resulted from centromeric fusion rather than elimination of chromosomes (Grossman et al., 1981). Acrocentric mono-armed chromosomes prevail in the karyotypes of larval B. luciopercae, 2n = 14, and larval A. isoporum sensu Wisniewski, 1959, 2n = 14 (Petkeviciute & Staneviciute, 2008). It is notable that the mean total length of haploid complements (TCL) of these two species does not exceed the TCL of C. crassum, despite different chromosome numbers.
It may be noted, however, that all of the heterophyids (Cryptocotyle and Acetodextra), bucephalids (Bucephalus and Rhipidocotyle), fasciolids (Fasciola), and zoogonids (Zoogonus) examined (7 species), as well as 1 species of a gorgoderid (Probolitrema) and 1 of a paramphistomatid (Gigantocotyle) have a basic number of 6 [perhaps thus indicating some relationship to the more primitive paramphistomatids (n = 7)]. The notocotylids (Notocotylus) have 7 chromosomes, but too few examples have been studied to determine the value of such counts. One species of an allocreadid (Bunodera), 1 gorgoderid (Gorgoderina), and 1 schistosomatid (Schistosomatium) have 7 chromosomes, in addition to the 2 amphistomids (Zygocotyle and Gastrothylax). All of the Schistosoma species studied show n = 8, as do 2 species of gorgoderids (Gorgodera and Phyllodistomum), 1 troglotrematid (Paragonimus), 3 species of allocreadids (Bunodera, Crepidostomum and Allocreadium), 1 species of a rhopaliid (Rhopalias), and 2 species of the reniferids (Staphylodora and Telorchis), in addition to the 2 species of paramphistomatids (Cotylophoron and Diplodiscus). Two species of the azygiids (Azygia and Proterometra), 2 species of the plagiorchids (Eustomas and Glypthelmins), 1 species of a spirorchid (Spirorchis), 1 pronocephalid (Macrovestibulum), 1 lecithodendriid (Brandesia), 1 monorchid (Asymphylodora), 1 hemiurid (Halepegus), and 1 reniferid (Auridistomum), in addition to 1 race of the paramphistomatid Diplodiscus (temperatus) have 9 chromosomes. Those having a basic number of 10 chromosomes are 1 species of a cyclocoelid (Cyclocoelum), 2 species of dicrocoelids (Brachycoelium and Dicrocoelium), 1 clinostomatid (Clinostomum), and 1 hemiurid (Isoparorchis). Only Heronimus of the paramphistomatids falls in this category. Four species of plagiorchiids (3 Pneumonoeces and 1 Plagitura), 1 echinostomid (Parorchis), 2 lecithodendriids (Acanthatrium and Loxogenes), and 15 species of reniferids (1 Dasymetra, 1 Lechriorchis, 1 Natriodora, 6 Neorenifer, 2 Pneumatophilus, 1 Renifer, and 3 Telorchis) have 11 chromosomes. In these cases again no direct relationship to the paramphistomatids can be noted in terms of chromosome number, although in terms of the presence of an increased number of chromosomes as indicating a possible primitive condition, these forms might be regarded as less specialized than others of the Digenea. It should be pointed out, however, that in many cases it is not only in the matter of actual number of chromosomes that similarities (relationships) may be indicated: total volume of chromatic material, shapes and sizes of the chromosomes, point of spindle attachment to individual chromosomes, and behavior during division may afford evidence of equal importance. It is also definite that sufficient differentiation occurs to facilitate identification of species. One species of Cephalogonimus has 14 chromosomes which may be a case of doubling of the usual number of 7 in this genus. If one follows the belief of Ciordia (1949), who doubts the existence of polyploidy among the Trematodes, this would be an example of extreme aneuploidy-duplication of individual chromosomes and not of the set as a unit. The matter of the presence or absence of the so-called heterochromosome ("sex chromosome") has not been determined in most of the trematode species examined, but in a few cases the recognition of two types of sex cells differing from each other in terms of the number, size, shape, volume or behavior of the chromosomal elements would seem to indicate that such sexual differentiation does occur. As examples we may mention the studies on Schistosoma and Schistosomatium. The earlier observations on Schistosoma haematobium, S. mansoni, and S. japonicum [Katsurada, 1904]; seemed to indicate that two types of sperm could be identified and that adult males possessed 15 and adult females possessed 16 somatic chromosomes. This would seem to mean that an X-O condition obtained in these forms. Other studies reported the numbers as 14 and 16, respectively, and were interpreted as showing the presence of a 2X + 12 and a 4X + 12 chromosome complex. Niyamasena (1940) in his studies on S. mansoni found the somatic number of chromosomes to be 16 in each sex, and could not find any evidence of the presence of recognizable sex chromosomes although the possibility of an X-Y condition could not be ruled out. Most recent studies support the finding of 16 chromosomes as the diploid number in all adults of all three of the above Schistosoma species and the inability to recognize sex chromosomes as being present [16 chromosomes are also present in each sex of S. mansoni carcariae [Bilharz, 1852]. Recent studies on Schistosomatium douthitti [Bilharz, 1852]; have presented 2 different interpretations of what is undoubtedly an example of the presence of distinguishable sex chromosomes. One study (Woodhead, 1957) indicates the presence of a single "X" chromosome in the male, while the other (Short, 1957) presents evidence of the heterogametic condition as prevailing in the female. Studies in this laboratory seem to substantiate this second interpretation. The somatic (diploid) number of chromosomes in each sex is 14, with the male showing a pair of large V-shaped chromosomes that are not matched in the female. In the latter case there is a single large V-shaped chromosome apparently paired with a single rod-shaped body. This rod-shaped chromosome does not appear in any of the male cells. It is interpreted as indicative of a ZZAA condition in the male and a ZWAA condition in the female. Short & Menzel (1957) During the present study three monogeneans and one digenean trematodes were investigated for cytological investigation.
7. b) Monogeneans
8. i. Diplozoon kashmirensis Kaw, 1950
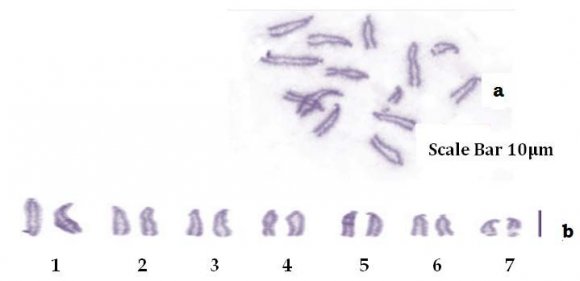
Analysis of mitotic metaphase spreads from ten specimens of Diplozoon kashmirensis showed that the karyotype of D. kashmirensis comprised 14 acrocentric chromosomes (2n=14; Fig. 1a). The karyotype formula can be summarized as 2n=14a (Fig. 1b). The longest pair is 14.13 ?m and the shortest pair is 6.21 ?m long (Table 2), the fundamental arm number (NF) = 7 and total chromosome length (TCL) is 72.57 ?m. Arm ratio of the complement ranges between 7.67-14.15 and the centromeric index ranges 6.60 to 12.28. Chromosome pair no. 2, 3 and 4 are nearly similar in their size and are very difficult to identify on the basis of chromosome morphology (Students T-test; P-value = 0.002; P<0.05), precise identification of second, third and fourth chromosome pairs is rather difficult because of the low degree of significance of length differences, but there is significant difference between chromosome pairs of 5 due to length difference between them. So, on the basis of absolute length and centromeric position, the chromosomes have been arranged in order of decreasing length in an ideogram (Fig. 2a,b) Karyotype Formula: (K) 2n=14= 14a
9. ii. Diplozoon aegyptensis Fischthal et Kuntz, 1963
The somatic complement of Diplozoon aegyptensis species revealed a diploid number of 2n = 14 (Fig. 3a) comprising first three pairs of chromosomes as metacentric and last four pairs of chromosomes as acrocentric in which a fundamental arm number (FN) equals 10 (Fig. 3b). The chromosomes range in length between 7.11 µm to 8.08 µm. The total length of the haploid complement equals 55.78 µm. Arm ratio of the complement ranges between 1.09-17.23 and the centromeric index ranges between 5.49-47.90 (Table . 3). On the basis of total length of chromosomes and relative length there is less significant difference between first three pairs of metacentric chromosomes (P=0.002 ; P<0.05 ; Students T-test) and significant difference between last four pairs of acrocentric chromosome pairs (P=0.001 ; P<0.001 ; Students Ttest). The absolute length and centromeric position of the chromosomes have been arranged in order of decreasing length in an ideogram (Fig. 4a,b ). Karyotype Formula : (K) 2n=14= 6m+8a iii. Diplozoon guptai Fayaz and Chishti, 1999 Diploid chromosome number of D. guptai is 2n=14 as revealed after examination of mitotic metaphase spreads from 13 specimens (Fig. 5a). Karyotype (Fig. 5b) included two metacentric (nos. 1 and 2); one submetacentric (no. 3); one subtelocentric (no. 4) and three acrocentric (no.s 5, 6 and 7) chromosome pair; the karyotype formula may be summarized as 2n=4m+2sm+2st+6a. The chromosomes are comparatively large; the smallest and the largest chromosomes measured 5.39 ?m and 8.02 ?m, respectively (for chromosome measurements, see Table 4). The number of chromosome arms (NF) is 20 and total chromosome length (TCL) is 47.25 ?m. Arm ratio of the complement ranges between 1.04-34.53 and the centromeric index ranges between 2.81-49.00 (Table . 3). Length difference between first three pairs are much less and there is less significant difference between them (P=0.002; P<0. G pairs, but there are significant length difference between four pairs of chromosomes (P=0.001 ; P<0.001 ; Students T-test). On the basis of absolute length and centromeric position, the chromosomes have been arranged in order of decreasing length in an ideogram (Fig. 6a,b). Karyotype Formula : (K) 2n=14=4m+2sm+2st+6a Karyological characterization of Monogenea species is a neglected subject. However, the study of karyotype of Diplozoon spp. was the first study in Kashmir Valley. Regarding diplozoids, 17 species have been studied cytogenetically to date (13 identified and 4 unclassified taxa, Table 4); karyotypes of only 9 additional monogeneans have been published (Benazzi and Benazzi Lentati 1976;Harris 1985;Rohde 1994;Cable and Harris 2002). As summarized in Table 4, all diplozoid species have chromosome sets comprising 14 acrocentric elements except two species which comprising 3 metacentric and 1 acrocentric elements. Taking into account a hypothesis that less advanced species of a group often have non-symmetric karyotypes (White 1973), this karyotype seems to represent an ancestral type (Koroleva 1969). Thus, Koroleva (1969) suggested that species with chromosome numbers lower than 14 might originate via Robertsonian centric-fusion translocations during evolution.
Four analyzed karyotypes of D. paradoxum, P. bliccae, P. nagibinae, and P. sapae were previously studied by Koroleva (1968aKoroleva ( , b, 1969)), and the data on number and classification of chromosomes fit well with the present results. However, our study has revealed new information on chromosome measurements of Diplozoon species of the Kashmir Valley. Koroleva (1968aKoroleva ( , 1969) ) showed no interspecific differences among species with 2n=14. She reported maximum chromosome length from 3 to 5 ?m in P. bliccae (syn. Diplozoon gussevi) and from 4 to 13 ?m in D. paradoxum (Koroleva 1968a). Our analysis revealed lower chromosome length, but such differences are likely related to different methodology used; it is known that air-dry and spreading techniques produce longer chromosomes than formerly used squashes (Reblanova et al. 2010). The most related congeners P. bliccae, P. nagibinae, and P. sapae (Matejusova et al. 2001(Matejusova et al. , 2004;;Gao et al. 2007) have equal number of 14 chromosomes of very similar morphology, all being acrocentric. However, our study showed that all the three species of Diplozoon examined contain 14 chromosomes with varying length of short and long arm and having different chromosome morphology. D. kashmirensis contains 14 chromosomes of which all are acrocentric (2n=14=14a) and have chromosome length ranging between 6.21-14.13 µm where as D. aegyptensis also contains 14 chromosomes but with different chromosome morphology in which the first three pairs are metacentric and rest of four pairs are acrocentric (2n=14=6m+8a) and have smallest and largest chromosome length between 6.84 and 9.78 µm. The third species D. guptai differs markedly in chromosome morphology (2n=14=4m+2sm+2st+6a) but it has nearly the same chromosome length as of D. aegyptensis i.e., having a short and long arm between 5.39 and 8.02 µm. These data correspond well with the above-mentioned hypothesis of Koroleva regarding an evolution of the D. paradoxum karyotype from an ancestral type with seven one-armed pairs. Regarding interspecific differences of different species of Diplozoon spp. they show variation of their relative lengths (Table 5). Thus, when comparing the relative length of Diplozoon kashmirensis with those of Diplozoon aegyptensis, the differences are not significant (T-value =-0.00, P-value = 0.999 and Pearson correlation =0.190; P-value = 0.683; P>0.05). Those of Diplozoon kashmirensis with Diplozoon guptai (T-Value =0.00, P-Value = 1.000 and Pearson correlation= -0.202, P-value = 0.664; P>0.05) again the differences are not significant and in Diplozoon aegyptensis compared to Diplozoon guptai (T-value =0.00, P-Value = 0.999 and Pearson correlation= -0.127 P-Value =0.787; P>0.05, here we again see that differences are not significant statically. Therefore, the noted differences in the relative chromosome lengths between the individual Diplozoon species cannot be used as a reliable criterion for establishing identification of the Diplozoon species. So, on the basis of centromeric index Diplozoon species can be used for the identification of different species. Thus, pericentromeric heterochromatin, occurring in acrocentric chromosomes of any of studied species with 2n=14, might be lost in the process of centric fusions. It is evident that further detailed cytogenetic study of subsequent diplozoid monogeneans will better reveal general routs of chromosome evolution within the relatively narrow group of interesting fish parasites.
10. a) Digenean Trematode
i. Clinostomum schizothoraxi Kaw, 1950 Colchicine treatment allowed us to examine enough number of metaphase plates with wellcontracted mitotic chromosomes. In this way, the number and morphology was determined with high accuracy. A diploid complement of 2n=20 was found in 43 dividing cells of Clinostomum schizothoraxi (Fig. 7 &8) collected from Schizothorax and Carassius spp. The chromosomes are large; the smallest measured 5.88 ?m and the largest 9.15?m (Table 5 & 6). Karyotype (Pmg. 4.37) included one metacentric (no. 1); two submetacentric (no.s 2 and 3); three subtelocentric (no.s 4; 5 and 6) and four acrocentric (no.s 7, 8; 9 and 10) chromosome pair; the karyotype formula may be summarized as 2n=20=1m+2sm+3t+4a. Number of chromosome arms (NF) was 26; total haploid complement length (TCL) was 77.12 ?m. Arm ratio of the complement ranges between 1.51-18.31 and the centromeric index ranges from 5.18-39.88 (Table 4 G absolute length and centromeric position the chromosomes have been arranged in order of decreasing length in an ideogram and the karyotype formula is; Karyotype Formula : (K) 2n=20=1m+ 2sm+3t+4a.
11. V. Discussion
Digenean Trematodes are karyotypically conservative, and their karyotypes tend to have the same number and closely related gross chromosome morphology of the genus and family (or even higher) taxonomic level. Most of the karyologically studied members of Digenean families are Troglotrematidae, Plagiorchiidae, Telorchiidae, Prosthogonimidae, and Lecithodendriidae. Information on the cytogenetics of G family Allocreadiidae, where some pairs measures up to 8 µm in length and smallest in the families Clinostomidae and Plagiorchiidae, in case of the present study the chromosomes are medium sized; the smallest measured 5.88 ?m and the largest 9.15 ?m. His work was mainly concerned with collecting cytological evidences to find an evolutionary mechanism in Platyhelminthes. However, Short and Menzel (1960) concluded that morphological changes accompanying separation of the genera seem to have been the results of translocations, inversions, deletions, and changes in the number of smaller chromosomes of Digeneans. The present results on karyotype of Clinostomum schizothoraxi, 2n=20, are in agreement with Britt (1947) who described 20 chromosomes of Clinostomum marginatum. Despite limitations of the method used, Britt (1947) established that small chromosomes are characteristic of Clinostomidae species and our data confirm these findings. Mitotic chromosomes of Clinostomum schizothoraxi are medium sized, up to 9.15 ?m. Notable workers like Cable (1931Cable ( , 1974)); Anderson (1935); Chen (1937); Rees (1937); Pennypacker (1936Pennypacker ( , 1940)); Markell (1943); Ciordia (1949;; Willmott (1950); Willey and Godman (1950); John (1956); Dhingra (1954a-c,1955a-c); Guildford (1955,1961); Short, (1955Short, ( , 1957) and Short and Menzel (1957, 1959; Sanderson (1959); Barton (1960); Greson (1958Greson ( , 1964)); Sharma et al. (1968a-b); Saksena (1969); Subramanyam (1973, 1975); Sharma et al (1974) and Jha (1975) have observed interesting cytological variations regarding the chromosome number and morphology in the order Digeneans.
Changes in chromosome form in digeneans trematodes were believed most commonly from centric fusion, pericentric inversions, changes in the amount of heterochromatin and euchromatin, and through other chromosomal rearrangements (Grossman & Cain, 1981). Often a likely route for the evolution of chromosomes within a family or genus can be visualised; key indicators include: karyotypic variation between related species whose diploid complements differ (Barsiene & Grabda-Kazubska, 1988a); atypically large chromosomes (Grossman & Cain, 1981); the relative lengths of chromosomes comprising the haploid genome (White, 1973); and the chromosome arm number (Mutafova, 1994). One of the most commonly observed evolutionary pathways occurring in the digenean genome results from a Robertsonian translocation.
12. VI. Conclusion
Studies of trematode material have been relatively numerous, and the results point definitely toward the desirability of continued efforts in this field of research. The discovery of the presence of heterochromosomes, particularly of the fact that some forms show the female as the heterogamic sex, is of especial interest and importance. Some definite taxonomic relationships are recognizable, but since in some cases they substantiate other types of evidence used in establishing phylogenetic position and in other cases the results seem to be contradictory, further observations are in order. Perhaps some of the apparent contradictions may be eliminated as our knowledge increases. Such definitely has been the case among the Turbellaria, as mentioned in the body of this paper. In the digenetic trematodes studied till to date, most variations in the chromosome numbers within a genus are seldom greater than + 1 or 2 bivalents. Thus the mechanism for an addition or deletion of the chromosome must operate at a low level or inefficient level in this group. This suggest that the differences in the have come about by a doubling of the whole sets of chromosome but by a gradual addition or losses. Each change which represents aneuploid condition becomes stabilized. When variation in the chromosome number exceeds 1 or 2 bivalents, it probably represents successive aneuploid conditions, each change followed by a period of stability in the new chromosome number.
13. VII. Acknowledgement
Volume XVI Issue I Version I







| report a similar condition |
| Chromos | Length of | Length of | Total | Arm Ratio | Relativ | Centro | Classification | |
| ome pair | short arm | long arm | Length/Abs | (L/S) | e | meric | ||
| number | (µm) 'S' | (µm) 'L' | olute | Length | Index | |||
| Length | (%) | (ci) | ||||||
| (µm) L+S | ||||||||
| 1 | 1.63 | 12.5 | 14.13 | 7.67 | 19.47 | 11.54 | Acrocentric T-Value = -25.39 | |
| 2 3 | 1.47 1.22 | 10.5 9.80 | 11.97 11.02 | 7.14 8.03 | 16.49 15.19 | 12.28 11.07 | Acrocentric Acrocentric | P-Value = 0.002 P<0.05 |
| 4 | 1.12 | 9.00 | 10.92 | 8.03 | 15.05 | 10.26 | Acrocentric | |
| 5 | 0.89 | 8.80 | 9.69 | 9.89 | 13.35 | 9.19 | Acrocentric T-Value = -10.88 | |
| 6 | 0.63 | 8.00 | 8.63 | 12.60 | 11.89 | 7.30 | Acrocentric | P-Value = 0.000 |
| 7 | 0.41 | 5.80 | 6.21 | 14.15 | 8.56 | 6.60 | Acrocentric | P<0.001 |
| Chromos | Length of | Length | Total | Arm | Relative | Centromeric | Classification | |
| ome pair | short arm | of long | Length/Absol | Ratio | Length | Index (ci) | ||
| number | (µm) 'S' | arm | ute Length | (L/S) | (%) | |||
| (µm) 'L' | (µm) L+S | |||||||
| 1 | 3.87 | 4.21 | 8.08 | 1.09 | 14.49 | 47.90 | Metacentric | T-Value = |
| 2 3 | 3.31 3.03 | 4.07 3.81 | 7.38 6.84 | 1.23 1.26 | 13.23 12.26 | 44.85 44.30 | Metacentric Metacentric | -20.56 P-Value = 0.002 |
| P<0.05 | ||||||||
| 4 | 0.93 | 8.85 | 9.78 | 9.52 | 17.53 | 9.51 | Acrocentric | T-Value = |
| 5 | 0.77 | 7.89 | 8.66 | 10.25 | 15.53 | 8.89 | Acrocentric | -14.82 P- |
| 6 | 0.51 | 7.42 | 7.93 | 14.55 | 14.22 | 6.43 | Acrocentric | Value = |
| 7 | 0.39 | 6.72 | 7.11 | 17.23 | 12.75 | 5.49 | Acrocentric | 0.001 |
| P<0.001 | ||||||||
| Year 2016 |
| Volume XVI Issue I Version I |
| D D D D ) |
| ( |
| There is less chromosome length differece between 2 & |
| 3; 4 & 5, 6, 7 & 8 and 9 &10 which are statistically less |
| significant (P<0.05 ; Students T-test). On the basis of |
| Chromo | Length | Length | Total | Arm | Relativ | Centromer | Classification | |
| some | of short | of long | Length/Abs | Ratio | e | ic | ||
| pair | arm | arm | olute | (L/S) | Lengt | Index (ci) | ||
| number | (µm) 'S' | (µm) 'L' | Length | h (%) | ||||
| (µm) L+S | ||||||||
| 1 | 3.21 | 4.84 | 8.05 | 1.51 | 10.44 | 39.88 | Metacentric | |
| 2 | 2.13 | 4.11 | 6.24 | 1.93 | 8.09 | 34.13 | Submetacentric P-Value = 0.020 P<0.05 | |
| 3 | 2.00 | 3.88 | 5.88 | 1.94 | 7.62 | 34.01 | Submetacentric | |
| 4 | 1.54 | 6.63 | 8.17 | 4.31 | 10.59 | 18.85 | Subtelocentric | P-Value = 0.012; |
| 5 | 1.33 | 6.52 | 7.85 | 4.90 | 10.18 | 16.94 | Subtelocentric | P<0.05 |
| 6 | 1.00 | 5.43 | 6.43 | 5.43 | 8.34 | 15.55 | Subtelocentric | P-Value = 0.011 |
| 7 | 0.84 | 8.31 | 9.15 | 9.89 | 11.86 | 9.18 | Acrocentric | P<0.05 |
| 8 | 0.71 | 8.13 | 8.84 | 11.45 | 11.46 | 8.03 | Acrocentric | |
| 9 | 0.56 | 7.84 | 8.40 | 14.00 | 10.89 | 6.67 | Acrocentric | P-Value = 0.010 |
| 10 | 0.42 | 7.69 | 8.11 | 18.31 | 10.52 | 5.18 | Acrocentric | P<0.001 |