1. I. Introduction
oagulase-negative staphylococci (CoNS) are part of normal commensals of the skin, anterior nares, and ear canals of humans [1]. Because of their relatively low virulence, they have long been considered as nonpathogenetic, and were rarely reported to cause severe infections. However, as a result of the combination of increased use of intravascular devices and an increase in the number of hospitalised immunocompromised patients, CoNS have emerged and are increasingly recognised as a major agents of clinically significant infection of the bloodstream and other sites [2,3,4,5,6,7,8].
Given the frequency with which multiple antimicrobial resistance is encountered, treatment of CoNS infections can be challenging and oxazolidinone: Line zolid has been considered the drug of choice for the management of infections caused by gram-positive organisms, including resistant organisms, such as methicillin-resistant Staphylococcus aureus, methicillinresistant coagulase-negative staphylococci (MRCoNS), vancomycin-resistant enterococci, and multidrugresistant Streptococcus pneumonia [9,10,11,12,13,14,15]. However, widespread use of linezolid recently has led to the emergence of CoNS isolates with decreased susceptibility to these agents further limiting therapeutic options for treatment of infections caused by these organisms [16,17,18].
In Nigeria, to date, Linezolid-Resistant Coagulase Negative Staphylococcus (LRCoNS) have not been reported, although there are no indications of the use of linezolid within the study area, it is recognized as one of the few drugs that have been reported to be effective in the treatment of infections caused by MRCoNS. In the current study, we determined nasal carriage rate of CoNS and antimicrobial resistance profile of these coagulase-negative staphylococci isolates with linezolid resistance that were recovered from apparently healthy undergraduate students in Niger Delta University. This study will however serve as a reference point data for nasal carriage rate and Linezolid antimicrobial profile of CoNS for the region.
2. II. Materials and Methods
3. a) Sampling Area
The study was carried out in Amassoma, a semi urban settlement in the Niger Delta and is home to the Niger Delta University with a student population of about 20,000. It is located on Latitude 4? 59' 09'' N and longitude 6? 06' 34'' E. Its land area is 2,682Km2 (1,036 sq miles) at an elevation/altitude of 9 metres. It is in an area of high humidity (mean: 300C) and temperature (average: 26.7? C with annual rainfall of about 1777mm.
The students sampled in this study were medical and nursing students of the university. They are of age: (range: 15-39, mean = 22), Sex: (Males: 124; Females: 276) and have stayed a period of 1 year minimum in the University
4. b) Sampling
Anterior nares swabs were collected in accord to protocols described by Rongpharpi et al [19]. A total of 400 nasal swabs were collected from anterior nares of apparently healthy subjects aseptically using a sterile swab sticks (Copan Diagnostics, Corona, CA, USA). Swabs were transported in Amies (Oxoid, England) transport medium to the Medical microbiology laboratory of the College of Health Sciences, Niger Delta University for bacteriological assay.
5. c) Isolation and Identification
In the laboratory, each swab was immediately inoculated onto Mannitol Salt Agar (MSA; Oxoid, England) plates and incubated at 37C for 24 h. The characteristic isolates were aseptically isolated and characterized using established microbiological methods that include colonial morphology, Gram stain characteristics, haemolysin production catalase, coagulase tests as well as DNase production [20]. The various isolates were identified to species level by employing standard microbiological methods [20,21]. Coagulase negative-Staphylococci isolates were confirmed through the use of the Staph identification 25 E (BioMeriux, France).
6. d) Antimicrobial Susceptibility Testing
The antimicrobial susceptibility pattern of all the isolates to Augmentin (30?g), Cefoxitin (30?g), Ciprofloxacin (5?g), Co-trimoxazole (25?g), Erythromycin (15?g), Gentamycin (30?g), Linezolid (30?g), and Tetracycline (30?g) all obtained from Oxoid (England) were determined using modified single disc diffusion technique in accordance to the guidelines of Clinical and Laboratory Standards Institute (CLSI, 2012) [22]. Briefly, standardized overnight culture of each isolate (containing approximately 106 cfu/ml) which was equivalent to 0.5 McFarland Standard was used to swab the surface of Mueller Hinton agar plates and excess drained off and dried while the Petri dish lid was in place. The standard antimicrobial discs were aseptically placed at reasonable equidistance on the inoculated plates and allowed to stand for I hr. The plates (prepared in duplicates) were then incubated at 37 0 C for 18-24 h. The diameter of the zone of inhibition produced by each antimicrobial disc was measured with a ruler in millimeters. Breakpoints and interpretative for susceptibility/resistance was based on the CSLI criteria [22]. We used the agar dilution method to further confirm the Linezolid MIC's (lowest concentration at which growth was inhibited) values of the linezolidnonsusceptible CoNS isolates. The MIC (?g/mL) interpretative standard for linezolid were those suggested by CLSI [22], (respectively: ? 4 susceptible, ? 8 resistant). The procedure was performed in duplicate on separate occasions, and the means of the duplicates were used. Staphylococcus aureus NCTC6571 was used as the quality control in each set of tests.
7. e) Statistical analysis
SPSS for Windows (version 20.0; SPSS) software was used for the analysis. Frequency distribution, mean, harmonic mean, standard deviation, analysis of variance (ANOVA) were determined. Categorical variables were compared by using Pearson's chi-squared test (?2) or Fisher's exact probability tests. P-values were calculated and P ?0.05 was considered statistically significant
8. III. Results
As depicted in Table 1, 227(56.8%) of the 400 studied subjects yielded Staphylococci growths. The overall carriage rates of Coagulase Negative Staphylococci was 136(34.03%).
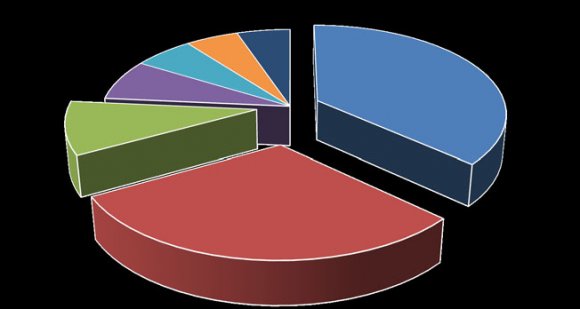
As shown in Figure 1, we identified and confirmed that the 136 CoNS strains belong to 7 species including S. epidermidis 50(36.76%) which is the most prevalent. This is followed by S. haemolyticus 41(30.15%), S. saprophyticus 13(9.56%), S. hominis 10(7.35%), S. cohnii 8(5.88%), while Staphylococcus lugdunensis and S. xylosus were 7(5.15%) each.
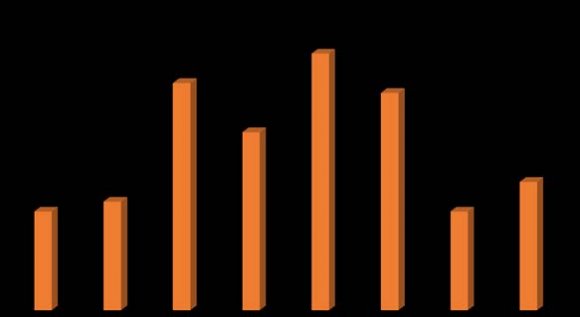
Figure 2, shows the antimicrobial susceptibility profile of the isolates. Overall 112(82.4%) of the 136 CoNS isolates showed resistance to Erythromycin, while resistance were 108 (79.4) The prevalence of multiple antibiotic resistance (MAR) of the isolates was investigated. One hundred and twelve (82.35%) of the isolates showed multiple resistance in varying degrees. Twenty-three (20.54%), 18 (16.07%), 26(23.21%), 22(19.64%), and 10 (8.93%) were resistant to 3, 4, 5, 6, and 7 antibiotics among the isolated strains respectively. Thirteen (11.61%) of the isolates were resistant to all the 8 antibiotics tested (Figure 3).
9. IV. Discussion
We conducted this study in order to determine the nasal carriage rate and antimicrobial resistance profile of CoNS strains isolated from the anterior nares of apparently healthy students of a tertiary institution in Wilberforce Island, Amassoma. The institution is situated in a semi urban area in Bayelsa-state in the Niger Delta. The result obtained from the present study will serve as a reference data for CoNS carriage rate. In addition, the study also gives an understanding into the patterns of antimicrobial resistance profile of these isolates in the locality.
The study revealed that 136 out of the 400 subjects examined were positive for CoNS in their anterior nares, indicating the nasal carriage rate of 34.03%. Earlier, report indicates the nasal carriage rate of CoNS to vary from 13% to 56% in different populations [13,23,24,25]. Though we observed lower figure in the present study, our findings is in comparison with the carriage rates documented by Morgenstern et al. [26] and Lebeaux et al. [27] in Portugal and France respectively. Contrast with our findings, higher nasal carriage rates have however been reported by Koziol-Montewka et al., 2006 in Poland (55.8%) [28], Campeotto et al. 2004 in Brazil (66.1%) [29], Akhtar 2010 inPakistan (73.3%) [30] and Abadi et al. 2015 in Iran (77.7%) [31]. Shibabaw et al. [32] attributed these differences to various microbiological methods (sampling techniques to culture media) employed, the local infection control standards and the local prevalence rate. Aside from these, it has been suggested that carrier rates might also be influenced by poor personal hygiene, poor environmental sanitation [32] and age-related dynamics of the study participants [1]. The low recovery rate of CoNS observed in the present study might be due to the fact that our subjects being medical and nursing students may have been involved in good hygiene practices with hand washing inclusive. On the other hand, as documented by Onasoga, et al., 2015 [33], they may have also been involved in self-medication or predisposed to the misuse of antibiotics.
The results showed that seven species of CoNS were identified. The most common species isolated was S. epidermidis 50(36.76%). The similar results were recorded in many studies [34]. Various studies have indicated most CoNS isolates obtained in the present study as responsible for infections that are of endogenous origin particularly among immunocompromised and individuals that are hospitalized [35,36,37,38,39,40].
Over the years, studies have shown that antimicrobial therapy causes marked symptom improvement and shortens the duration of illness associated with Staphylococci infections. Before now, various types of antimicrobial agents have been efficacious in the management of Staphylococci infections, but options for treatment of these diseases are becoming restricted due to the appearance of multidrug-resistant strains of CoNS. There has been global concern about the emergence of antimicrobial resistance in common pathogens of community as well as nosocomial infections and CoNS have demonstrated a pattern of progressively increasing resistance to antibiotics worldwide [41,42,43,44,45,46,47,48]. The results obtained from the present study indicates that 112(82.35%) of the 136 isolates from this environment are multiply resistant to antibiotics, (Figure 3). In comparison, the pattern of multidrug resistance demonstrated here has been described among CoNS isolates in different part of the world which includes Switzerland [26], India [49], Iran [31,34], China [44] , USA [50] ,France [27], Pakistan [30], Italy [51] and Poland [28].
The antibiotic susceptibility pattern of the isolates shows that Gentamycin was the most effective among the CoNS, followed by augmentin, in that order (Figure 2). When compared with existing report, the 22.8% resistance of the CoNS isolates to Gentamycin in this finding corroborates the report of Ma et al. [48] and is different with report of Al-Muhanna et al. [34] that 32% of CoNS isolates were resistant to Gentamycin, while Roopa and Biradar [49] and Zhanel et al. [52] reported 0.0% and 78.8% resistance of these pathogens to Gentamycin respectively. So gentamycin is the only drug in this study that is proven to be effective for CoNS. One of the reason for this high susceptibility seen in this study may be that gentamycin appears to be infrequently used as it administered by injection, a dosage form which is far less amenable to selfmedication than orally administered antibiotics in this locality [53].
On the other hand, the high susceptibility to augmentin observed in this study is in sharp contrast to existing reports (31.6% versus 70%; P < 0.0001) by Abdalla et al. [54] and Akinkunmi and Lamikanra [55] that 70% and 62.4% resistance of CoNS to augumentin respectively. Nonetheless, the present findings corroborates the report of Roopa and Biradar [49]. One of the reason that could be adduced to low resistance observed in this study may be that augumentin, though an orally administered antibiotics, seems to be rarely abused by individuals in the locality because of its exorbitant price (about 10USD) for a packet in a locality where people live below 1USD per day.
The antimicrobial resistance profile of CoNS isolated in this study indicated that 58.8% of the isolates were resistant to Cefoxitin [MR-CoNS]. This result is higher than earlier report [49,55], and, lower than report made by Al-Muhanna et al., [34], Maet al. [48] and Koksal et al [56]. However, it is similar to the report made by Lebeaux et al. [27] among the organisms isolated in their respective studies. Reports have documented that resistance to Cefoxitin by disc diffusion can be used for the detection of MRSA strains in routine testing [57] because Cefoxitin is regarded as a potential inducer of the system that regulates mecA gene [58]. For this reason, the resistant of our isolates which were found to be resistant to Cefoxitin are considered resistant to methicillin (58.8% MR-CoNS).
During the susceptibility test in the present study, one of our limitations was excluding Vancomycin, the drug considered efficacious for MRSA and MRCoNS, from the test because of unavailability of its commercial disc. Nonetheless, Delorme et al. [59] reported the exclusion of vancomycin from their study because vancomycin may produce erratic results in disc diffusion susceptibility test [59]. However, even with the absence of vancomycin susceptibility test, the result of this study can be compared with the findings of several outcomes including [60,61,62,63] which established that linezolid is a drug that is as effective as vancomycin. Both antibiotics do not just have similar failure and success rates but adverse effects as well [61,64].
Approximately, 69.1% of the CoNS isolates showed high resistance to trimethoprim/sulfamethoxazole in this study. This is similar to what has been reported by Koksal et al. [56] and Akinkunmi and Lamikanra [55] in Turkey and Ile-Ife, Nigeria respectively and many other researchers, a finding correlated to that by Ma et al [48] and Abadi et al. [31]. This could be due to the fact that this drug is very commonly available in our setting and is also indiscriminately used for prophylaxis by individuals with symptoms of Upper Respiratory Tract infections (URTI) and Urinary Tract Infections (UTI). The study by Paul et al. [65] showed zero resistance to trimethoprim/sulfamethoxazole to Staphylococcus aureus in Nigeria in 1985, while Gu et al. [10] showed 29.4% trimethoprim/sulfamethoxazole in Greece. This is worthy of mention and comparison. It shows that trimethoprim/sulfamethoxazole resistance has increased prodigiously over the prevailing years.
The majority of our CoNS isolates were highly resistant to erythromycin (82.4%), and the high rate (79.4%) of resistant to tetracycline and Ciprofloxacin (55.1%), found in this study is worrisome considering the ability of these organisms to spread easily by direct or indirect person-to-person contact with resultant therapeutic complications and considering that ciprofloxacin has been identified as the drug being the most efficaciousanti-infective drug in Nigeria [43,66].
Combating the increase in mortality and morbidity due to therapeutic failures in the treatment of multidrug resistant Staphylococci infections, particularly those that are methicillin and vancomycin resistances, gave rise to the need for newer efficacious therapeutic options leads to the discovery and approval of oxazolidinone antibiotic: linezolid by FDA in 2000 as an attractive alternative to vancomycin and MRSA [60,67]. It is tragic that barely one year of its introduction into treatment regime for multidrug resistant Gram-positive organisms, the first resistant among Enterococcus faecium, was reported [68] and Tsiodras et al. [69] reported the first Linezolid resistant Staphylococcus aureus in a US patient. Since then, linezolid-resistant S. aureus and CoNS have been detected in separate cases and outbreaks worldwide [10,70,71].
Currently, 48.5% of CoNS isolated from the present study were found to be linezolid resistant. Making this finding one of the highest resistance rate recovered among CoNS isolates in Nigeria and amongst those recorded globally. This study revealed that S. epidermidis, S. haemolyticus, S. cohnii, S. saprophyticus, S. hominisisolates, were resistant to linezolid in 52%, 50%, 48.78%, 46.15%, and 40% respectively, while S. lugdunensisand S. xylosus showed 42.86% resistance to linezolid each (Table 2). To confirm this resistivity, we decided to carryout Minimum Inhibitory Concentration tests on these isolates as suggested by CLSI 2012 [22], and the outcome showed that all our linezolid resistant isolates had MICs >256µg/mL.
Previous studies have shown various resistance profiles of CoNS to linezolid. For example, Morgenstern et al. [26] in Switzerland and [44] in China reported 0% resistance to linezolid respectively. However, globally, surveillance studies report <1% of CoNSas linezolid resistant. But, a study conducted by Potoski et al. [72] in the USA, observed Linezolid resistance in about 4.0% of MRCoNS isolates. Another study conducted in USA by Helio and colleagues reported LRCoNS in 0.1% [50]. Similarly, incidence of 0% was reported by Al-Muhanna et al. [34] from Iraq. Ugwuet al. [66] reported 0% LRCoNS in Southern Nigeria. The high incidence of linezolid resistance in the organisms isolated in this study is not expected since this antibiotic is not broadly used within the study environment, this is worrisome and is worthy of note. Particularly that linezolid is not routinely prescribed and administered in our locality. More so that the drug is very difficult, if available in our market, in other words, its availability for misuse or selfadministration as reported among other antibiotics is not anticipated [73]. So, this high resistance recorded must be of concern to practitioners and public health, more so, that these organisms live in association with other organisms in their ecological niche and can disseminate these resistances to other organisms within the environment [73].This collaborates reports made by Garcia et al. [74] that horizontal transmission of linezolid resistance could pose a serious threat, because the cfrgene can also be transmitted between species, such as from S. epidermidis, which although not pathogenic, could become a reservoir for resistance genes and that this mode of transmission becomes more difficult to prevent and stop than those of nosocomial spread that are usually controlled with standard measures, such as isolation, barrier precautions, and antibiotic restriction On the other hand, these organisms and their antimicrobial resistancehave been documented to be associated with opportunistic infections and can be transferred from these individuals to the patients, hospital environments and the community [41,75,76] making it a life-threatening organism which may lead to increase mortality and morbidity, particularly among colonised individuals, immunocompromised and HIV patients.Staphylococcal resistance to linezolid (LZD) is said to be mediated through ribosomal mutations (23S rRNA or ribosomal proteins L3 and L4) or through methylation of 23S rRNA by the horizontally transferred chloramphenicol-florfenicol resistance (Cfr)plasmidborne ribosomal methyltransferase that catalyzes methylation of A2503 in the 23S rRNA gene of the large 50S ribosomal subunit, conferring resistance to chloramphenicol, florfenicol, and clindamycin [9,77,78,79,80,81,82]. The first cfr-mediated, linezolid-resistant clinical isolate of MRSA was reported in 2007 by Tohet al. [83]. More recently, linezolid resistance has been identified due to acquisition of a natural resistance gene, cfr, so the high resistance of the present study CoNS isolates to linezolid might be due to acquisition of resistance to chloramphenicol, as chloramphenicol is one of the antibiotics that are readily available and most abuse, misuse and self-medicated in our locality. However, this assumption would be further investigated.
10. V. Conclusion
The study findings indicate the usefulness of investigation of CoNS colonisation of the nasal mucosa the primary ecological niche for these microorganisms in order to better understand the epidemiology of this phenomenon, but also to develop prevention measures and treatment strategies in case of established infections among predisposed individuals.
11. VI. Acknowledgements
We are grateful to Ogobiri Gloria Tamaraemomoemi, Tiemo, and Pereyan Cynthia for Collection of the samples and the school students for participating in this study.
12. Volume XVI Issue III Version I
| Staphylococci colonising anterior nares | |||
| Age | Male | Female | No (%) Isolate |
| 15-19 | 4 | 6 | 10(7.35) |
| 20-24 | 30 | 49 | 79(58.09) |
| 25-29 | 24 | 13 | 37(27.21) |
| 30-34 | 4 | 3 | 7(5.15) |
| 35-39 | 3 | 0 | 3(2.21) |
| Total | 65 | 71 | 136(100) |
| S. xylosus |
| (n=7) |