1. I. Introduction
enal cell carcinoma(RCC) accounts for more than 2% of cancers in humans worldwide [1,27]. It is the seventh most common malignancy in male and 12 th most common malignancy in female [2,28]. Many researchers have stated that renal cell carcinoma (RCC) is not a single disease but rather, a group of several disease entities [3,4,10]. In 2004 WHO classified RCC into different histopathologic types which is showed in table 1: The classification of renal cell carcinoma into subtypes has become of interest because of the association with prognosis [10]. Different tumor behavior and aggressiveness related to histologic subtypes and some others well-established parameter according to Fuhrman grade (tumor size and stage) [6,7,27]. Clear cell carcinoma also known as conventional renal carcinoma is the most common subtype, accounting for 65% of RCC [8,9]. Papillary and chromophobe renal carcinoma comprise 25% of RCC [8,9]. Collecting duct is a rare subtype, accounting for less than 1% of all RCC [5]. Patients with papillary renal carcinoma or with chromophobe renal carcinoma have a higher 5-year survival rate than those with conventional renal carcinoma of the same stage [2,4,5]. However, collecting duct carcinoma have the worst prognosis, with a 5-year survival rate less than 5% [5]. CT imaging posing a diagnostic dilemma for the practicing physician because it can provide detailed information about tumor itself and weather it has extended into perinephric fat or renal vein [10]. So it can play an important role in treatment planning.
2. II. Materials and Methods
3. a) Patients
A computerized search of our institution ' s medical records dated between February, 2014 and February, 2016 generated a list of 64 patients who had undergone nephrectomy for renal cell carcinoma. Of these 64 patients, the diagnosis for 39 patients with a pathologic diagnosis of clear cell carcinoma and 25 patients with non -clear cell carcinoma(6 with papillary cell carcinoma, 3 with chromophobe cell carcinoma, 2 with pelvicalyceal urothelial carcinoma, 2 with pelvicalyceal urothelial papillary carcinoma, 2 with Wilms' tumor, 2 with sarcomatoid RCC,1 with clear cell papillary carcinoma, 1 with clear cell sarcoma, 1 with malignant rhabdoid tumor, 1 with leiomyosarcoma, 1 with renal cell carcinoma associated with X11.2 dislocation TF3 fusions, 2 with angiomyolipoma and 1 with oncocytoma). 2 patients of angiomyolipoma and 1 patient of oncocytoma were excluded due to their benign cherecteristics. Therefore, 22 patients of nonclear cell carcinoma were included in our study. For the clear cell carcinoma (n=39; men 23
4. b) CT examination
All patients underwent pre-operative plain CT and triphasic DCE-CT examinations using a dual-source CT scanner (Somatom Defination; Siemens, Germany) and with our standard renal mass protocol tailored to each scanner. CT images were obtained during patient breath holding with following parameters -gantry rotation time:0.33s ; tube potential:100kV p ; effective tube current:100mA ; pitch:1.2 ; collimation:32mm x 0.6mm ; beam collimation:64mm x 0.6mm; slice thickness:5mm and intersection gap:5mm. All patients received oral contrast materials 30 minutes before CT. Unenhanced images were acquired before the intravenous injection of contrast media. After administrating contrast agent (Ultravist, 1.5 ml/kg) with a power injector at a flow rate of 3.0ml/sec, corticomedullary, nephrographic and excretory phase images were obtained at 25-45sec, 60-90sec, 240-300 sec respectively. All images were sent to our enterprise-wide picture archiving and communications system to be interpreted on workstations.
5. c) Image analysis
Tow experienced genitourinary radiologists who were aware that patients were being evaluated for renal lesions, but they were blinded to any other clinical, pathologic or imaging findings. Before, image interpretation, the readers met and agreed on the CT features to be used to characterize renal masses and a data collection form. They reviewed the CT scans at picture archiving and communications system. They compared patient age, sex; size and shape of tumor; margin whether well-defined or ill-defined; location; presence or absence of calcification, hemorrhage, necrosis or any cystic change; presence or absence of thrombus in renal vein or inferior vena cava, ascites and lymphadenopathy; pattern (homogeneous or heterogeneous) and degree of enhancement (hyperdese, hypodense or isodense). For comparison of location ,they described it in three patterns : Location1 (tumor located either right or left side); Location 2 [tumor involved upper, middle, lower pole or mixed (involvement of more than one pole)]; Location 3 [involved cortex, medulla ,pelvis or mixed(involvement of more than one layer)].
6. d) Statistical analysis
Analysis were performed by using SPSS17.1 software. We used the Pearson X 2 test to compare the distribution of features across the two groups. A P value less than 0.05 indicated a statistically significant difference.
7. III. Results
Of 64 renal lesions included in this study, 39 were clear cell RCC S, 25 were non-clear cell RCC S . 3 of 25 non-clear cell renal carcinoma (2-angiomyolipoma and 1-oncocytoma) were excluded as their benign behavior. So, total number of non-clear cell carcinoma were 22. Patient presented for CT examination at the CT laboratory from February, 2014 to February, 2016. The CT images were analyzed retrospectively.
Baseline characteristics for each of the groups are presented in table 2:
Volume XVII Issue 1 Version I Year 2017 ( D D D D ) D Table 2: Characteristic ccRCC non-ccRCCThere were no significant differences, when we compared age; sex; shape of tumor; presence or absence of (necrosis, hemorrhage) in between two groups.
But, when we analyzed the degree of enhancement (hyperdensity, isodensity, hypodensity) in arterial (corticomedullary) and venous (nephrographic) phases showed significant difference. In arterial phase, most of clear cell RCC (21 of 39, 53.8%) showed hyperdensity, whereas none of non -ccRCC (0 of 22,0%) showed hyperdensity. The P value was 0 (P<0.05). In venous phase, ccRCC showed more hyperdensity or isodensity (9 and 4 0f 39, 23.1% and 10.3% respectively) than non-ccRCC (0 and 1 of 22, 0% and 4.5% respectively). Almost all of the non-clear cell RCC ( 21 However, we did not get any significant difference, when compared degree of enhancement in delayed phase (excretory phase). Table 3: shows the comparison of degree of enhancement in different phases in between ccRCC and non-ccRCC. The pattern of enhancement (homogeneous or heterogeneous) showed significant difference. Nonclear cell carcinoma (19 of 22, 86%) showed more heterogeneous enhancement pattern than that of clear cell carcinoma (21 of 39,53%). The P value was 0.012 (p<0.05).
When, compared location of tumor (whether it involved upper, middle or lower pole of kidney), we found that 15 of 39 (38.5%) ccRCC were located in middle pole; but most of non-clear RCC (15 of 22,68%) did not show any specific polarity predilection. They involved two or all of 3 poles. The P value was 0.001(p<0.05) Most of the clear cell RCC (33 of 39,84.6%) showed involvement of medulla, whereas most of the non clear cell RCC (20 of 22,90%) did not show such predilection for a specific layer. They involved more than one layer. the P value was significant (p =0). But when, we compared involvement of pelvis, we found that non-ccRCC (2 of 22,9%) showed more pelvis involvement than ccRCC (0 of 39,0%).
Calcification is more common in non-clear cell RCC 27% (6 of 22) than clear cell RCC 7% (3 of 39).The p value was significant (p=0.038). In our study, we also made comparison in between non-clear cell RCC and clear cell RCC with hypovascular tumor. We found significant p values when we compared size, location, pattern of enhancement and presence or absence of necrosis in between these two types.
The mean size of hypovascular ccRCC was (3.92±1.89)cm, whereas mean size of non-ccRCC was (6.18±2.89)cm. The P value was 0.023(P<0.05).
Non-clear cell carcinoma (19 of 22, 86.4%) showed more heterogeneous enhancement pattern than hypovasculer clear cell RCC (2 of 8, 25%). P value was 0.003 (p<0.05).
When, we compared presence or absence of necrosis, we found that, necrosis was more common in non-clear cell RCC (18 of 22, 81.8%) than ccRCC with hypovascular tumor (2 of 8, 25%). The P value was significant (P=0.007).
In our study, we also found that most of nonclear cell RCC layers (20 of 22, 90.9%) showed mixed involvement of different layer of kidney (cortex, medulla and pelvis) that means no specific predilection for any layer, whereas most of hypovascular ccRCC (4 of 8, 50%) showed involvement of medulla. The p was 0 (<0.05).
Table 5: Summaries difference in between hypovascular ccRCC and non-ccRCC:
However, there were no significant differences in between hypovascular ccRCC and non -ccRCC, when we made comparison for shape (round or lobulated), rim (clear or unclear), presence or absence of (hemorrhage, calcification and metastasis). The P values were (>0.05).
8. IV. Discussion
Now-a-days, the incidence of renal cell carcinoma is increasing due to increasing risk factors (obesity, smoking) and utilization of modern imaging techniques [11][12][13]29]. A majority of renal tumors are incidentally diagnosed on medical imaging, that's why most of them are asymptomatic, small in size and present at an earlier stage [14,27]. It is important to discriminate clear cell RCC from non-clear cell RCC because of ccRCC is generally considered to have a worse prognosis and is treated differently than other subtypes [15][16][17][18]27]. Several study has been done previously to differentiate clear cell RCC from non-clear cell RCC by using imaging modalities. The most consistent finding was that, degree of enhancement was the most valuable parameter for differentiation of renal cell carcinoma subtypes. Clear cell RCC S enhance to a greater degree than other subtypes of malignant lesions [8,10,[19][20][21][22]. Some researchers stated that the strong enhancement of conventional renal carcinoma is caused by it , s rich vascularity and alveolar architecture at histologic examination [4,10,23]. Our study consistent with these study. In this study, we found ccRCC (53.8%) showed more hyperdensity than that of non-ccRCC(0%). Most of non-ccRCC (95.5%) had hypodensity in all phases.
However, when we compared pattern of enhancement, most of clear cell RCC (53.5%) showed heterogeneity, which agree with other studies related with pattern of enhancement of ccRCC [8,10]. But, when we made comparison of heterogeneity in between ccRCC and non-ccRCC, we found that, non-ccRCC were more heterogeneous than ccRCC. This may be because of larger size of non-ccRCC S which tended to show heterogeneity due to propensity of hemorrhage, necrosis and calcification [24][25][26]. At microscopic examination, all tumors with homogeneous enhancement were mainly composed of solid elements, whereas all tumors with heterogeneous enhancement had solid elements, necrosis, hemorrhage and calcifications.
When, we made comparison in between clear cell RCC S and non-clear cell RCC S for the presence of calcification, we found that calcification was significantly more in non-ccRCC (27%) than that of ccRCC S (7%). Calcification suggests a higher 5-years survival rate [3,10].
To our knowledge, it is the first study which made comparison in between two groups for the predilection of pole(upper ,middle,lower) and for the involvement different layer(cortex, medulla, pelvis). We found that ccRCC showed more middle pole predilection (84%) than that of non-ccRCC(27.3%), whereas majority of non-ccRCC showed mixed polarity means involvement of more than one pole (68.2%). ccRCC (84.6%) had predilection for involvement of medulla, whereas most of the non-ccRCC(90%) had no specific predilection for any layer, they involved more than one layer. In case of pelvis involvement, non-ccRCC (9%) showed more pelvis involvement than that of ccRCC(0%).
In this study, we also made comparison in between ccRCC which showed hypovascularity and non-ccRCC. The number of ccRCC with hypovascularity was 8. Non-ccRCC (86.4%) were more heterogeneous than hypovascular ccRCC(25%). We also found that, necrosis was more common in non-ccRCC (81.8%) than hypovascular ccRCC(25%) and involvement of pelvis was more common in non-ccRCC(9.15%) than hypovascular ccRCC(0%). Hypovascular ccRCC (50%) showed predilection for involvement of medulla and most of non-ccRCC(90.9%) did not show any specific predilection for involvement of cortex, medulla and pelvis, rather than they showed involvement of more than one layer(mixed involvement).
Our study had few potential limitations. First, our study was retrospective study. Second, we did not measure CT value of different kinds of tumor. did not compare clear cell renal carcinoma with any other specific type of non-clear cell renal carcinoma. We compared ccRCC with as a whole others non-ccRCC. So, it may be a limitation. The study population of nonclear cell renal carcinoma was small in number.
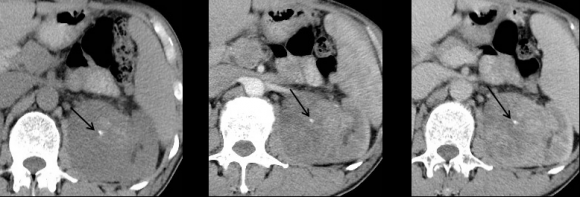
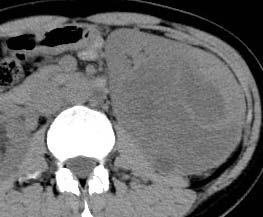
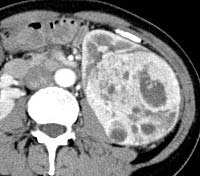
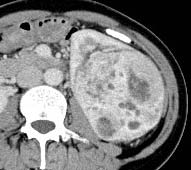
| Clear cell (conventional)RCC |
| Multi locular clear cell RCC |
| Papillary RCC |
| Chromophobe RCC |
| Carcinoma of collecting ducts of Bellini |
| Renal medullary carcinoma |
| X P 11 translocation carcinoma |
| Carcinoma associated with neuroblastoma |
| Mucinous tubular spindle cell carcinoma |
| Unclassified RCC |
| Source-Reference 30 |
| Differences in Contrast-Enhanced CT Features between Clear Cell Renal Carcinoma and Non-Clear Cell | |||
| Renal Carcinoma | |||
| Characteristic | ccRCC | non-ccRCC | |
| Sex | |||
| male | 23 | 16 | |
| female | 16 | 06 | |
| Mean age(years) | 54.59+/-11.05 43.82+/-23.7 | ||
| Mean size(cm) | 5.08+/-3.57 | 6.18 +/-2.89 | |
| Hemorrhage | 03 | 02 | |
| Necrosis | 24 | 18 | |
| Calcification | 03 | 06 | |
| Rim | |||
| clear | 12 | 10 | |
| Unclear | 27 | 12 | |
| Shape | |||
| round | 34 | 17 | |
| irregular | 05 | 05 | |
| Homogeneous | 18 | 03 | |
| Heterogeneous | 21 | 19 | |
| Hyperdense | 21 | 00 | |
| Hypodense | 08 | 21 | |
| Isodense | 10 | 01 | |
| Location1 | |||
| Right | 21 | 13 | |
| Left | 18 | 09 | |
| Location-2 | |||
| Upper | 07 | 01 | |
| Middle | 15 | 06 | |
| Lower | 09 | 00 | |
| Mixed | 08 | 15 | |
| Location-3 | |||
| Cortex Medulla | 02 33 | 00 00 | D D D D ) D |
| Pelvis | 00 | 02 | ( |
| Mixed | 04 | 20 | |
| Metastases | 04 | ||
| Types | Pattern of enhancement | Location 2 | Location 3 | Calcification | ||||||||
| Homo | Hetero | 1 " | 2 " | 3" | 4" | 1" | 2" | 3" | 4" | Yes | No | |
| ccRCC | 18 | 21 | 7 | 15 | 9 | 8 | 2 | 33 | 0 | 4 | 3 | 36 |
| Non-ccRCC | 03 | 19 | 1 | 6 | 0 | 15 | 0 | 0 | 2 | 20 | 6 | 16 |
| P value | P=0.012 | P=0.001 | P=0 | P=0.038 | ||||||||
| NOTE: | ||||||||||||
| Pattern of enhancement | Necrosis | Location 3 | |||||||
| Homo | Hetero | No | Yes | 1" | 2" | 3" | 4" | ||
| Hypo ccRCC (n=8) | 06 | 02 | 06 | 02 | 02 | 04 | 00 | 02 | |
| Non-ccRCC (n=22) | 03 | 19 | 04 | 18 | 00 | 00 | 02 | 20 | |
| P value | P=0.003 | P=0.007 | P=0.00 | ||||||
| Note: | |||||||||
| Hypo ccRCC=Hypovascular clear cell renal carcinoma ,Non-ccRCC=Non-clear cell renal carcinoma, | |||||||||
| Homo=Homogeneous, Hetero=Heterogeneous | |||||||||
| Year 2017 | Location 3 (1"=Cortex, 2"=Medulla, 3"=Pelvis,4"=Mixed) | ||||||||
| Volume XVII Issue 1 Version I | |||||||||
| D D D D ) D | |||||||||
| ( | |||||||||