1. I. Introduction
besity is a disease characterized by dysregulated accumulation of fat in the body, which is associated with health risks due to its relationship with various metabolic complications. It is simultaneously a disease and one of the most important risk factors for other chronic non -communicable diseases, such as cardiovascular diseases and Diabetes mellitus (Pi-Sunyer et al., 1997; Halpern and Mancini, 1999;Halpern et al., 2000;Fortes et al., 2006).
For the treatment of this disease the daily insertion of pharmacological and / or nonpharmacological therapies such as physical exercises, change of eating habits, surgical procedures and medications, respectively, is recommended.
In an attempt to aid in the treatment of obesity, there are currently drugs with direct and / or indirect weight-loss properties such as those that inhibit appetite (catecholaminergic), which increase satiety (serotonergic), those that decrease fat absorption and those that increase burning of fat (Guyton e Hall, 1997; Pi-Sunyer, 1997; Halpern e Mancini, 1999; Radominski, 2010).
Among these drugs, Orlistat® is of recent use in the treatment of obesity, which has a mechanism of action different from the others because it inhibits the lipases of the gastrointestinal tract, which are responsible for the cleavage and subsequent absorption of fatty acids (Drent e Veen, 1993;Drent et al., 1995;Zhi et al., 1995;Zhi et al., 1996;James et al., 1997).
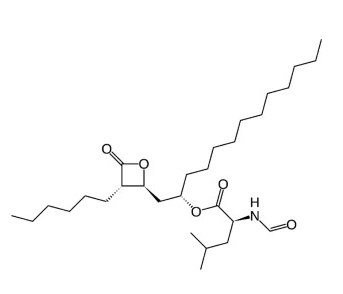
Also known as tetrahydrolipostatin, it is a specific inhibitor of gastric and pancreatic lipases, which are important for aiding the digestion of fats in the diet (Drent et al., 1995). This drug is chemically synthesized from a hydrogenated derivative of the lipostatin produced by Streptomyces toxytricini (Drent e Veen , 1993; Zhi et al., 1994; Amatruda e Welle, 1995). With the following structure (Fig. 1):
The evaluation of the carcinogenic risk / benefit ratio should always be performed before prescribing a drug (Brambilla et al., 2011;Brambilla et al., 2012).
Therefore, this work aims to contribute to a riskbenefit projection of the use of this drug, demonstrating the possible genotoxic effects of this treatment. The objective of this study was to evaluate the mutagenic potential of Orlistat® in meristematic cells of Allium cepa; Quantify the mutagenic effects of Orlistat® in the test system used; contribute to the elucidation of information on probable adverse effects caused by the indiscriminate use of Orlistat®.
2. II. Methodology
Organic onion bulbs were purchased locally with reliable source. The dry external scales were removed without damaging the root area and the central O K parenchyma of the bud crown was also removed by circular incision to increase the uptake and uniformity of budding and root growth. These bulbs were washed in running water for about 20 minutes. Carefully, the roots of the bulbs were exposed with the samples in covered glass beakers to prevent light from entering, so that only the central parenchyma of the bud crown was in contact with the samples. For each sample analyzed, five onion bulbs were used and placed in contact with the samples for 24 hours. The negative control was performed in the same manner using distilled water and ethanol in the ratio 1: 1 (solvent) (Rank. and Nielsen, 1993;Kruger, 2009;Cuchiar et al., 2012).
The standard orlistat test concentrations for the experiments were 60 mg/L, 360 mg/L and 500 mg/L. These concentrations were selected on the basis of the doses considered subdose (where the dose described is lower than the dose at which the drug reaches therapeutic effect), therapeutic dose (from 60 to 120mg, 3 times a day) until overdose (where the dose described is higher than the dose at which the drug achieves therapeutic effect, and may reach toxicological effect (Zhi et al., 1995). The positive control was Paracetamol® at 800 mg / L concentration.
After growth, the roots immersed in the samples were measured and then fixed in Carnoy's solution (acetic acid and ethyl alcohol, in the concentration of 3: 1) for 12 hours. After fixation, the roots were washed in distilled water for five minutes and stained on slides. For this, the roots were stained with acetic orcein dye in the dilution of 2% orcein and 45% acetic acid. The root tips were cut and heated for one minute in counting with the dye. Then, the roots were placed on slides covered by coverslips and one drop of acetic orcein dye was added between slide and cover slip. Subsequently, the root was crushed by gentle pressure. The observation of the slides was performed under an optical microscope with a 100x objective, counting 5000 cells, observing the mitotic indexes and the chromosomal and mitotic changes (Ribeiro and Grotzner, 2012;Dias, 2014).
The calculation of the mitotic index (MI) and the index of chromosomal and mitotic aberrations (ICMA) occurred according to the following equations: MI = number of cells in mitosis x 100 ÷ total number of cells observed ICMA = number of altered cells x 100 ÷ total number of cells observed For statistical analysis, the ANOVA test was used, with significance level ? = 0.05, using the statistical package GrafPad Prism 5.0.
3. III.
4. Results
The characterization of the genotoxicity and cytotoxicity of Orlistat® was performed by root growth analysis of Allium cepa, in order to evaluate the inhibition of root growth, mitotic index (GMI) and mitotic and chromosomal abnormalities index (MCAI).
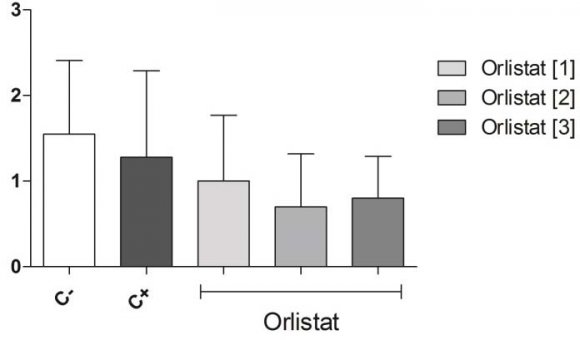
The results of the analysis of variance by the ANOVA test of root growth are described in Fig. 2. It was possible to verify that the root growth of a strain in the negative control did not present statistically significant difference of the means obtained in the roots treated with the three concentrations of the drug, so Orlistat® did not interfere with the growth of onion roots.
5. Fig. 2: Root growth of Allium cepa
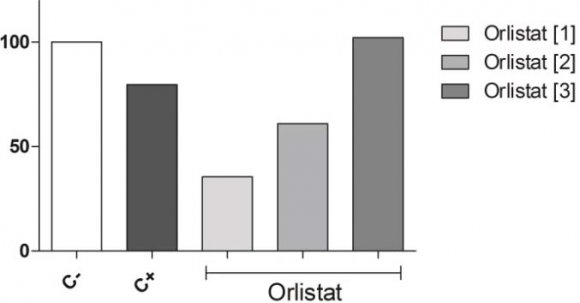
In relation to MI, when comparing the negative control and the treatments, in the three concentrations the drug significantly reduced the MI in the two lowest concentrations, as can be observed in Fig. 3.
6. Fig. 3: Mitotic Index
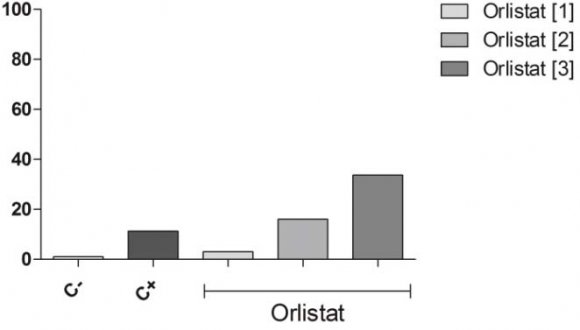
Orlistat® significantly increased ICMA when compared to the negative control. This increase had a dose-response effect (Fig. 4).
7. IV. Discussion
In the study of Lopes and Vicentini (2002) with mouse bone marrow cells, Orlistat® showed no mutagenic effect at the concentrations tested (0.2, 0.4 and 0.6 mg/mL). Concentrations tested in the above work are considerably lower than those tested in this study.
Based on this survey of the mutagenic potential of the Orlistat® drug in Allium cepa meristematic root cells, the number of mutations was high, as was a study with Drosophila melanogaster, in which Orlistat® was tested at the standard cross-Enzymatic basements) and improved cross-fertilization (with metabolic activation). At the standard crossing, the drug did not show genotoxic effects, but at the improved crossbreeding it was genotoxic, demonstrating that Orlistat® has an indirect genotoxic effect on D. melanogaster, suggesting that cytochrome P450 enzymes interfere with the genotoxicity of the compound. On the other hand, when co-administered with doxorubicin, Orlistat® modulated the action of this agent (Orsolin et al., 2012). Moreover,in carcinogenicity tests in Drosophila melanogaster, Orlistat® did not induce tumors, nor did it modulate the action of Mitomycin C in relation to tumor formation (Orsolin et al., 2012;Menendez et al., 2005), Orlistat® showed antitumor effect in breast cancer cells. However, in another study Orlistat® showed genotoxicity in human lymphocytes in the presence of caffeine by in vitro comet assays, was induced DNA damage prior to the repair mechanism (Chakrabart et al., 2016).
Therefore, as genotoxicity may be related to carcinogenesis, it is necessary to monitor chronic medications to make a profile with regard to the possible side effects produced by them and thus serve as support to ensure health safety oxcf people who use these medicines. Therefore, further research should be carried out in order to broaden our understanding of the genotoxicity of Orlistat®.