1. I. Introduction a) Origin Background
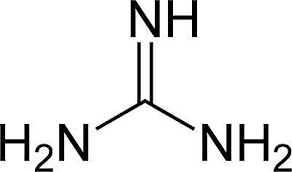
n 1920s, guanidine based compounds were firstly known with their efficacy as anti-hyperglycemic agents [1] , yet their origins go back to 1600s, when a plant; Galega officinalis, was commonly used in European Folklore to decrease the symptoms of what it's known today as type II diabetes (TY2D). [2] [3] Although this plant, also known as Goat's Rue or French Lilac, was found, in late 1800s, to be full of guanidine and galagine; isoamylene guanidine, [4] [5] [6] [7] however the efficacy of guanidine to lower blood glucose levels was not proven in animals till 1918.
This invention struggled some challenges as the toxicity of guanidine decreased its chance as a potential hypoglycemic drug, and the discovery of safer guanidine based structure compounds was somehow not succeeded by scientists, as well. Moreover, that the era of insulin discovery and availability by that time downplayed guanidine effectiveness role in diabetes treatment. [] Through what the world had witnessed of crises in 1900s, Researches did not stop investigating G. officinalis; there were still eyes on galagine as safer anti hyperglycemic agent than guanidine.
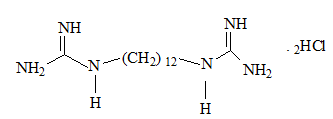
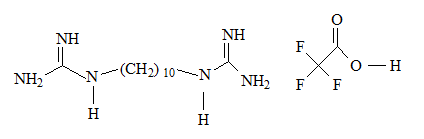
However, that was not for too long with the presence of diguanide compounds; decamethylene diguanide (Synthalin A ® ) and dodecamethylene diguanide (Synthalin B®) in the clinical use by the same period of time.
These diguanide compounds were more tolerated than guanidine compounds, but their hepatic toxicity reports in 1930s were the cause of not prescribing them any longer by medical professionals. [8] Although many guanidine compounds were synthesized in 1929, but biguanide derivates did not see the light till the later of 1950s. Phenoformin was discovered by Urgan and Shapiro and it was introduced to the medical usage in 1958 with DPI ® . [9] While Buformin was discovered by Mehnert. [10] In spite of these two proved greater efficacy than our unique compound, the lactic acidosis incidences that follow the use of them resulted in withdrawing them from the market in 1970s. [11] [12] [13] Metformin; Dimethyl-biguanide, the only bi-guanide remained in TY2D remedy, was developed by Jean Sterne in France. His results were published in 1957 and he was the one who named metformin as "Glucophage"; glucose eater. [14] [15] Yet, American Food and Drugs Administration did not approve on metformin usage in the U.S market till 1995. [16] b) Bi-guanide chemical development Author ?: Damascus University. e-mail: [email protected] [17] Their chemical and physical properties are shown in Table 1.
2. Metforimn Buformin Phenoformin
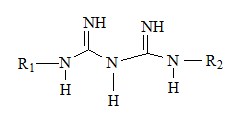
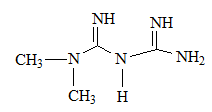
Metformin is the safest biguanide derivates in use by TY2D patients. [19] As can be inferred from metformin structure, it is a bi-guanide derivate, no (CH 2 ) n chain separates between the two guanidine groups. R 1 , R 2 are small alkyl groups (-CH 3 ).
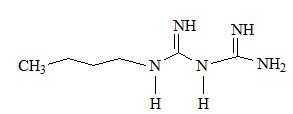
On the other hand, di-guanide derivates have been withdrawn from the market due to their adverse to toxic effects; Synthalins for their hepato-toxicity, while phenoformin and buformin; bi-guanide derivates, for their lactic acidosis incidences. [16] As can be seen from their structures, having (-CH 2 -) 10 chain, (-CH 2 -) 12 chain separating between two guanidine groups as there are respectively in Synthalin A and Synthalin B, or (Ar-CH 2 -CH 2 -) moiety as it is in phenoformin, might be responsible for the undesirable effects of these compounds. However, their antihyperglycemic efficacy were stronger than metformin.
This can indicate an inquiry that does shortening (CH 2 ) n ; n<10, or having other small or cyclic moieties on the both terminal NH 2 can take a part in these compound efficacy or decrease the adverse effects biguanide compounds struggled with?! This hypothesis is still in question.
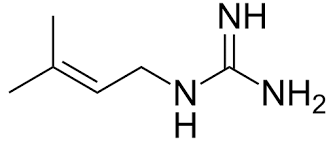
Recent research has proven that a number of other biguanide hydrochloride derivates also have anti diabetic activity. One of them, actually has a dioxymethyl benzene moity in its chemical Skelton. Structures of these compounds are shown in fig (5). [20] Shapiro had prepared metformin from the reaction between dimethylamine hydrochloride and dicyano diamide at 120-140 o C in 4 hrs time with 69% yield. [21] New route was used in this synthesis. This ecologically safe protocol using microwaves; 540W, 92% by optimum use of energy and tiny amounts of reacted materials. Fig ( 6) [22] Its mechanism of action does not differ from phenoformin's. They both increase AMPK activation in hepatic cells. [5] [25] [26] Recent research demonstrates that their pharmacodynamics play deeper role interacting with cellular bioactivity. they interact with Hepatic Mitochondrial Respiratory Chain Complex H + giant pump). [27] [28] [29] This results in decreasing the cellular production of ATP. [30] In both ways, Energy sources in hepatic cells are reduced resulting in enhancing insulin receptor sensitivity toward insulin. [31] That makes the main peripheral targeted cells; skeletal muscle, and hepatic cells, stop glycogenesis operation and produce more insulin receptors and Glucose Transporter 4 (GLUT-4) to augment glucose inwardly transportation. [32] [33] [34] [35] ii. Pharmacokinetics
No information was provided about biguanide derivates' pharmacokinetics but for Metformin. It is 50-60 % absorbed after an oral single dosage of (500. 850, 1000) mg. Its protein binding percentage is negligible. It is eliminated unchanged in the urine. [36] [37] Its half-life is 6.2 hours with duration of action 8-12 hours.
3. iii. Superiority
Hundreds of efficacy studies verified metformin superiority to the rest of his family members, and other chemical groups, used in Type II diabetes therapy. [38] [39] Likewise, toxicology experiments affirmed its narrow toxicity margin. Metformin advantage\ risk scale brought it to be the first drug in the guideline of initial remedy of Type II diabetes. [40] [41] As glucose blood levels become uncontrollable by time due to disease progression in TY2D and the life styles in many communities facilitates in this falling According to American Diabetes Association, metformin is the first-line drug for treatment of T2D patients in the world. Metformin can be considered Sugar Regulator rather than anti-hyperglycemic agent. [23] [24] Above all that, this ancient-modern small molecule can be used in adolescents and childhood, as TY2D since 1990 started to be caused not only in adults but also in children. [47] [48]
4. e) Side Effects and Other benefits
Metformin has no significant side effects with its labeled dosage. [49] The most frequently reported adverse events were digestive disturbance such as stomach ache, diarrhea, and vomiting. It also may cause headaches.
However, these undesired effects can be reduced when metformin is taken during meals, or as extended release pharmaceutical forms; metformin XR. [50] [51] Furthermore, metformin reduces mortality compared to other anti-diabetic agents. [52] This magical bi-guanide compound distinguished itself from other oral anti hyperglycemic agents side effects. Its usage does not lead to clinical hypoglycemia, lactic acidosis, or changes in physical examinations. Yet, rare lactic acidosis reported in some cases where over dosage was taken. [53] [54] In other words, Metformin magnificent side effect in its modest losing weight assistant made it likable to be prescribed by medical professions for obese TY2D patients. [55] [56] [57] This significant usage was especially observed in obese children with or without Type II diabetes (TY2D) compared to diet. Proposed mechanism of this effect is by reducing glucose absorption levels. [58] [59] [60] [61] [62] This herbal origin drug is also used in poly cystic ovary. [63] [64] Latest researches deepens its effectiveness in Cancer treatment, as well. [65] [66] [67] Besides to that, metformin has proven anti stress efficacy that led it to be used in inflammatory cases, and anti-aging factor, too. [68] back, many oral anti hyperglycemic agent combinations with metformin presented in the drug market. [42] [43] [44] Metformin combination with Sulfonyl-urea is very common. In fact, this double combination has proven more efficacy than if each drug was solely taken. Glucovans ® is the trade name of metformin and glyburide combination. [45] [46] Volume XVII Issue III Version I
Year 2017 ( D D D D ) BFurthermore, clinical studies verified its role in decreasing cardiovascular mortality by decreasing cholesterol blood levels and macro vascular cells' formation. [20] [69] [70] Metformin proved its positive role in metabolic syndrome patients, as well as it enhances neuron system health. [71] [72] [73] i. Contraindications
Heart failure is considered a potential contraindication for metformin usage in T2D treatment. Though lactic acidosis incidences are rare with metformin, yet this metabolic disturbance might be fatal in heart failure patients as it leads to hypo-perfusion. [74] [75] Despite this potential risk, yet FDA did not announce heart failure as contraindication for metformin. [76] Because metformin is eliminated unchanged in the urine, renal failure is another metformin contraindication. However, the level of renal dysfunction was not determined in which to be considered risky for metformin usage or not to avoid lactic acidosis incidences. [77] [78] [79] ii. Drug Interactions On the long term of taking, Metformin decreases the absorption of vitamin B 12 . [80] [81] Renal eliminated drugs such as digoxin, ranitidine, trimethoprime and many others also has interaction with metformin way of elimination.
iii. Prescriptions in the Market Though metformin was shadowed from the time it was synthesized; 1920s, passing by its development in 1950s, till it was approved by FDA in 1995; nowadays, Metformin is categorized as essential drug in the World Health Organization's (WHO) lists for TY2D treatment. [82] Bristol-Myers Squibb Company ; an American pharmaceutical global company, got the protection patent of marketing metformin under the trade name Glucophage till 2002. [83] Its prescriptions extended 50 million filled in the US alone, and Bristol-Myers Squibb earnings of this investment reached peak sales of US$2.7 billion in 2001. [84] By the time the protection patent expired, many companies such as Lubrizol, Zydus Pharmaceuticals and others compete for marketing approved generics such as Glucophage, Glucophage XR, Riomet, Fortamet, Glumetza, Jentadueto, JANUMET® XR, Synjardy®, INVOKAMET?.
Due to this strong market completion, It is expected that metformin market value increase 2.1% in 2021 compared with its market value in 2014. It is estimated that metformin market value in 2014 was 660 million USD. As global statics indicate, this might reach 720 million USD in 2021.
5. II. Conclusion
We tried in this review to shed light on biguanide discovery history and the chemical differences among this group compounds that are in a way or another responsible of their characteristics.
However, We look forward for more researches to be done in this field in aim to have as modified structures as it can be done to have better antihyperglycemic agents with less side effects and proven efficacy.
6. Volume
| Compound Name | M. Weight | Log P | pK a | Hydrogen bound Donner count | Hydrogen bound acceptor count | Dosage mg\day | Rel. potency |
| Synthalin A | 370.23 | N\A | N\A | 5 | 7 | N\A | N\A |
| Synthalin B | 357.368 | N\A | N\A | 4 | 4 | N\A | N\A |
| Buformin | 157.221 | -1.2 | N\A | 3 | 1 | N\A | N\A |
| Phenoformin | 241.723 | -0.83 | N\A | 4 | 1 | N\A | N\A |
| Metformin | 129.167 | -0.5 | 12.4 | 3 | 1 | 500\3 | N\A |
| microvascular and macrovascular disease in | |
| patients with type 2 diabetes mellitus. Arch Intern | |
| Med 2009, 169: 616-625. | |
| 71. Vitale C, Mercuro G, Cornoldi A, Fini M, et al: | |
| Metformin improves endothelial function in patients | |
| with metabolic syndrome. J Intern Med 2005, 258: | |
| 250-256. | |
| 72. Spasic MR, Callaerts P, Norga KK: AMP-Activated | |
| Protein Kinase (AMPK) Molecular Crossroad for | |
| Metabolic Control and Survival of Neurons. | |
| Neuroscientist 2009, 15(4): 309-316. | |
| 73. Van der Heide LP, Ramakers GM, Smidt MP: Insulin | |
| signaling in the central nervous system: learning to survive. Prog Neurobiol 2006, 79: 205-221. 74. Sulkin TV, Bosman D, Krentz A. Contraindications to metformin therapy in patients with NIDDM. Diabetes | Year 2017 |
| Care. 1997; 20: 925-9. | |
| 75. Evans JM, Ogston SA, Emslie-Smith A, Morris AD: | |
| Risk of mortality and adverse cardiovascular | |
| outcomes in type 2 diabetes: a comparison of | |
| patients treated with sulfonylureas and metformin. | |
| Diabetologia 2006, 49: 930-936. | |
| 76. Food and Drug Administration. Product label | |
| approval: metformin. 2006. http://packageinserts. | |
| bms.com/pi/piglucophage.pdf | |
| 77. | |
| D D D D ) B | |
| ( |