1. I. Introduction
ntibiotic resistance is when bacteria develop the ability to resist the bactericidal or bacteriostatic effects of one or more antibiotic class (multidrug resistance (MDR)) (1). This resistance is most commonly noted in intensive care units (ICUs), which is due to the widespread use of antibiotics in these units compared to the other hospital departments (2). A study found that the incidence of ICU nosocomial infections worldwide was between 5%-30% (3). According to the national healthcare safety network report in the United States (US); age, comorbid diseases, duration of hospitalization, length of ICU stay, immune status, and disease severity are all considered host risk factors for developing nosocomial infections in ICUs (4). In a study done on southern and eastern Mediterranean hospitals, overuse was one of the factors associated with increased antibiotic resistance (5). However, antibiotic resistance differs between ICUs in different countries due to various reasons including the different patterns of antibiotic use, the variation in infection control policies, and the effect of local resistance data in some countries directing the suitable antibiotic therapy which in turn leads to various outcomes on patients and healthcare systems (6). A previous study done in King Abdulaziz Medical City (KAMC), Riyadh Saudi Arabia from 2004-2009 including only Gram-negative bacteria (GNB) in the adult ICU, Acinetobacter baumannii, followed by Pseudomonas aeruginosa, Escherichia coli (E.coli), Klebsiellapnemoniae, Stenotrophomonasmaltophilia, and Enterobacter were the most commonly isolated organisms (7). During the study period, the resistance of different common pathogens was increasing significantly. Globally, the efficacy of antibiotics against various ICU pathogens is decreasing over the past few years (7). Therefore, continuous surveillance studies should be conducted locally to observe the emergence of different bacterial resistance patterns, as there are clear differences between international and national data.
2. II. Methodology
A retrospective cross-sectional study was carried out of GNB from the adult ICU of King Abdulaziz Medical City (KAMC) between 2010 and 2014. The yearly antibiogram data obtained from the ICU department was used to seek the percentage of GBN resistance against specific antibiotics. The result of 7600 GNBisolates were interpreted according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI). Gram-negative bacilli were identified to the species level and AST performed using an automated system (The VITEK® 2 system ,BioMariex, France) and the antimicrobial susceptibility testing confirmed by E-Test (AB Biodisk). Only one isolate per patient per year was included in the analysis. The following antimicrobial agents were tested either by the breakpoint method (with the vitek 2 system) or by the ETEST method using the following antibiotics on (Muller Hinton Agar Plate): amikacin ampicillin ceftazidime ceftriaxone ciprofloxacin gentamicin imipenem trimethoprim-sulfamethoxazole Quality control was performed by testing these same antimicrobials on E.coli ATCC 25922, E coli ATCC 35218, P aeruginosa ATCC 27853, and Enterococcus faecalis ATCC 29212 to check the thymidine level on Muller Hinton Agar.
The proportion of susceptible isolates was calculated as the sum of susceptible organisms (neither intermediately susceptible not resistant) relative to the total number of organisms tested. Multidrug resistance was defined as resistance to three or more antimicrobials (imipenem, ceftazidime, ciprofloxacin, pipracillin-tazobactam, and/or an aminoglycoside). The trend in the susceptibility rate over a 5-year period (between 2010-2014) was calculated and analyzed to identify a statistically significant increasing or decreasing trend using chi-square for linear trend analysis. Associations between categorical variables were tested using the chi-square test. The percent of change of antibiotic susceptibility was calculated as the difference between the later (e.g. 2014) and earlier (e.g. 2010) susceptibilities percentages divided by the earlier one. All P values were two-tailed. P value <0.05 was considered as significant. The data were analyzed using the Statistical Package for the Social Sciences, Version 20.0 (IBM Corporation, Armonk, NY, USA).
3. III. Results
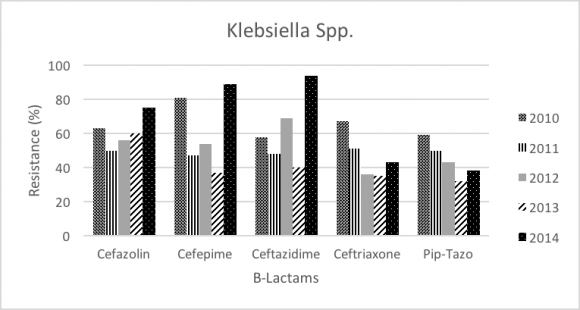
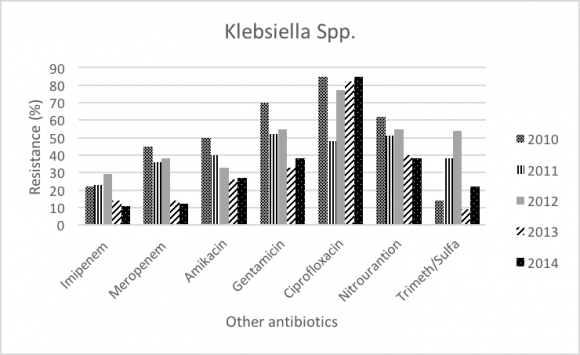
Throughout the study period (2010-2014), Klebsiella was the most commonly GNB in ICU (20.26%), and number of isolates in 2010 was 22.5% and 21.4% in 2014. Klebsiella resistance was significantly increased for Cefepime (81% to 89%; P-value= 0.001), and Ceftazidime (58% to 94%; P-value<.0001). In addition, Klebsiella resistance faced significant decrease in Ceftriaxone (67% to 43%; P-value<.0001), Carbapenems (meropenem 22% to 11%; P-value<.0001, and Imipenem 18% to 14%; P-value<.0001), Aminoglycosides (Amikacin 45% to 12%; P-value<.0001, and Gentamicin 50% to 27%; P-value<.0001), and Fluoroquinolone (Ciprofloxacin 70% to 38%; P-value<.0001).
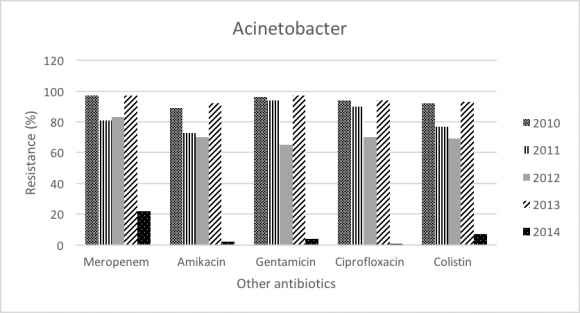
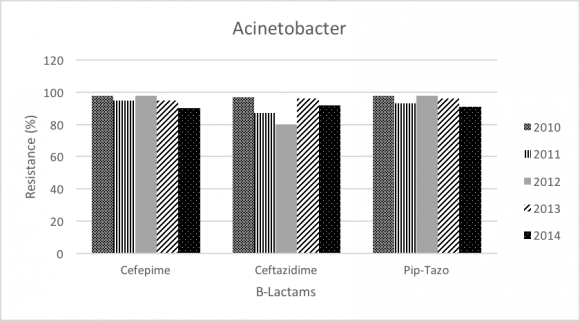
Acinetobacter baumannii accounts for 17.97% of all GNB, and number of isolates were 17.04% in 2010 and 11.8% in 2014. Acinetobacter baumannii demonstrated increase in resistance toward Carbapenems (Imipenem 87% to 92%; P-value<.0001); however, resistance pattern seems to be decreasing in Meropenem (97% to 92%; P-value= 0.473), Colistin (22% to 7%; P-value<.0001), and Amikacin (81% to 77%; P-value= 0.121).
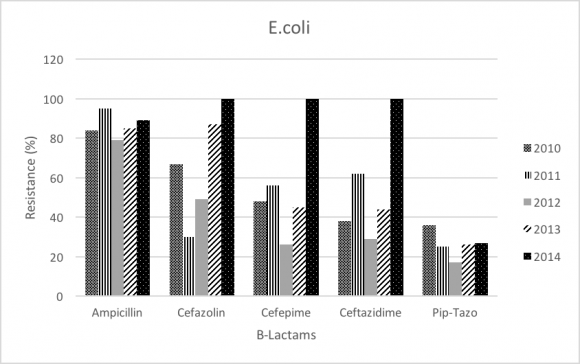
E.coli was 9.6% of all GNB, and the number of isolates were 10.17% in 2010 and 9.32% in 2014.The resistance pattern seems to be increasing in betalactam antibiotics including Cefazolin (67% to 100%; P-value<.0001), Cefepime (48% to 100%; P-value<.0001), Ceftazidime (38% to 100%; P-value<.0001), and fluoroquinolone (Ciprofloxacin 65% to 70%; P-value= 0.271). On the other hand, E. coli resistance rate decreased for Piperacillin-tazobactam (36% to 27%; P-value= 0.276), and no resistance difference in imipenem and meropenem throughout the study period (0%; P-value=0.325).
Enterobacter isolates account for 4.5% of GNB, and number of isolates were 5.4% in 2010 and 4.3% in 2014. The resistance for some beta-lactam is increasing especially in Cefepime (47% to 69%; P-value=0.260), Ceftazidime (56% to 95%; P-value=0.002). Moreover, Carbapenems (meropenem 3% to 5%; P-value=0.670, and Imipenem 6% to 23%; P-value<.0001) showed slight increase in the resistance pattern against Enterobacter. Aminoglycosides (Amikacin 41% to 2%; P-value<.0001, and Gentamicin 31% to 8%; P-value<.0001), and fluoroquinolones (Ciprofloxacin 31% to 19%; P-value=0.016) showed decrease in resistance toward Enterobacter.
4. IV. Discussion
Most of the hospital-acquired infections are related to invasive procedures and devices which are commonly seen in ICUs (8). The resistance pattern is most commonly noted in ICUs due to the widespread use of antibiotics in these units compared to the other hospital departments (2), and 70% of these infections were caused by GNB. (3). The increase in multidrug resistant organisms were shown to negatively affect the patient safety in which they can prolong the hospital stay, increase mortality rates, and health care costs (9).
This 5-year surveillance study is aimed to continue assessing the pattern of antibiotic resistance in GNB from adult ICU KAMC, Riyadh. As the annual antibiogram system were used in 2004 to 2009 to analyze the most common organisms and pattern of antibiotic resistance in our ICU. During the previous study period Acinetobacter baumannii revealed significant increase in resistance toward imipenem (45% to 90%), meropenem (67% to 90%), ciprofloxacin (78% to 90%), and amikacin (88% to 94%). Pseudomonas aeruginosa resistance markedly increased in 2007 specifically to carbapenems (34% to 74%), and ciprofloxacin (33% to 51%). E.coli showed significant increase in resistance to Cefuroxime (26% to 64%), ceftazidime (24% to 54%), cefotaxime (24% to 54), cefepime (23% to 50%), and ampicillin (64% to 73%). S marcescens showed increase in resistance toward cefotaxime (27%% to 68%), ceftazidime (9% to 65%), and pipracillin-tazobactam (20% to 36%). Enterobacter resistance was markedly increased to ceftazidime (66% to 95%), cefotaxime (66% to 94%), and pipracillintazobactam (49% to 65%).
In our study (2010-2014) the most commonly isolated GNB wereKlebsiella pneumoniae, Acinetobacter baumannii, Escherichia coli, and Enterobacter. In contrast, the previous surveillance (2004-2009), Pseudomonas aeruginosa and Stenotrophomonas maltophilia were considered as part of the most common GNB. Our data showed significant increase in resistance of Klebsiellatoward beta-lactams antibiotics especially ceftazidime (58% to 94%), and significant decrease in resistance in meropenem (22% to 11%). Most of the isolated Klebsiella showed increased betalactamase activity, and the rate of Extended-spectrum beta-lactamases (ESBL) isolates increased from 12% in 2004 to 21.4% 2014. This increase might be due to implementation of new screening program in 2007. In the previous study, there was one case of carabamenase-resistant klebsiella. However, carbapenems are still considered very effective agent against Klebsiella and the resistance pattern seems to be decreasing during our study period (meropenem 22% to 11%, and Imipenem 18% to 14%). Despite that, carabapenamase resistant isolates should be taken into consideration due to their potential dissemination. The trend of the overall resistance pattern is illustrated in figure-1 and figure-2.
In addition, Acinetobacter baumannii resistance was significant toward imipenem (87% to 92%). For that, the resistance pattern seems to be progressing over the period of 2004-2014. Furthermore, meropenem showed a slight decrease in resistance (97% to 92%) that is not statistically significant. Colistin remains the most effective antibiotic against Acinetobacter baumannii and our study showed significant decrease in the resistance (22% to 7%). As the treatment options for carbapenem resistant Acinetobacter baumannii are limited and challenging, colistin might be used empirically in the setting of our ICUs. The trend of the overall resistance pattern is illustrated in figure 3 and figure 4.
Most of E. coli isolates exhibited ESBL activity, and resistance is significantly increased in all betalactams antibiotics especially ceftazidime (38% to 100%); while the previous surveillance study showed E. coli resistance to ceftazidime (24% to 54%). Pipracillintazobactam showed slight decrease in resistance (36% to 27%); however, this decrease is not statistically significant. All our ESBL-producing isolates were susceptible to carbapenems. There was no significant increase in the rate of E. coli ESBL from 2004 (9%) to 2014 (9.34%). The trend of the overall resistance pattern is illustrated in figure-5 and figure-6.
Enterobacter exhibited significant increase in resistance mostly toward ceftazidime (56% to 95%), and carbapenems showed unique increase in resistance to imipenem (6% to 23%). However, meropenem increase in resistance was not statistically significant. Aminoglycosides remain the most effective antibiotic against Enterobacter with amikacin being broadly active. The trend of the overall resistance pattern is illustrated in figure-7 and figure-8.
5. V. Conclusion
Our study concluded that Gram-negative bacterial resistance is still a major issue in KAMC, Riyadh adult. ICU. The most commonly isolated GNB were Klebsiella pneumoniae (20.26%), Acinetobacter baumannii (17.97%), Escherichia coli (9.6%), and Enterobacter (4.15%). Carbapenems is considered the most effective agent for E. coli and Klebsiella ESBL. Aminoglycosides is the most effective agent for Enterobacter, and Colistin is the drug of choice for most cases of Acinetobacter baumannii. This significant resistance observed in ICU is mostly due to the overuse of broad-spectrum antibiotics, prolonged patient stay, and variation in infection control policies. Thus, the importance of collaboration between the ICU, infection control, infectious disease departments is very essential to substantially decrease the resistance rates. Furthermore, establishment of local database of antibiogram across the whole kingdom of Saudi Arabia will aid in the improvement of treatment strategies and guidelines based on unit-specific data.
6. Volume XVII Issue IV Version I
| Antibiotic | Resistance (%) in 2010 | Resistance (%) in 2014 | P-value | Trend |
| Beta-Lactam Antibiotics: | ||||
| Cefazolin | 67% | 100% | <.0001 | ? |
| Cefepime | 48% | 100% | <.0001 | ? |
| Ceftazidime | 38% | 100% | <.0001 | ? |
| Ceftriaxone | 45% | 59% | <.0001 | ? |
| Pip-Tazo | 36% | 27% | <.0001 | ? |
| Other Antibiotic Groups: | ||||
| Imipenem | 18% | 14% | <.0001 | â??" |
| Meropenem | 22% | 11% | <.0001 | â??" |
| Amikacin | 45% | 12% | <.0001 | â??" |
| Gentamicin | 50% | 27% | <.0001 | â??" |
| Ciprofloxacin | 70% | 38% | <.0001 | â??" |
| Nitrofurantoin | 85% | 85% | <.0001 | ? |
| Trimeth/Sulfa | 62% | 38% | <.0001 | â??" |
| Year 2017 | ||||
| Volume XVII Issue IV Version I | ||||
| D D D D ) | ||||
| ( | ||||
| Antibiotic | Resistance (%) in 2010 Resistance (%) in 2014 | P-value | Trend | |
| Beta-Lactam Antibiotics: | ||||
| Cefepime | 98% | 90% | 0.001 | â??" |
| Ceftazidime | 97% | 92% | 0.298 | â??" |
| Pip-Tazo | 98% | 91% | 0.026 | â??" |
| Other Antibiotic Groups: | ||||
| Imipenem | 87% | 92% | <.0001 | ? |
| Meropenem | 97% | 92% | 0.473 | â??" |
| Amikacin | 81% | 77% | 0.121 | â??" |
| Gentamicin | 81% | 69% | 0.010 | â??" |
| Ciprofloxacin | 97% | 93% | 0.232 | â??" |
| Colistin | 22% | 7% | <.0001 | â??" |
| Antibiotic | Resistance (%) 2010 | Resistance (%) 2014 | P-Value | Trend |
| Beta-Lactams antibiotics | ||||
| Cefazolin | 67 | 100 | <0.0001 | ? |
| Cefepime | 48 | 100 | <0.0001 | ? |
| Ceftazidime | 38 | 100 | <0.0001 | ? |
| Pip-Tazo | 36 | 27 | 0.276 | â??" |
| Others antibiotics | ||||
| Amikacin | 9 | 11 | 0.617 | ? |
| Gentamicin | 37 | 34 | 0.908 | â??" |
| Ciprofloxacin | 65 | 70 | 0.271 | ? |
| Nitrourantion | 8 | 19 | 0.002 | ? |
| Trimeth/Sulfa | 75 | 75 | 0.809 | _ |
| Antibiotic | Resistance (%) 2010 | Resistance (%) 2014 | P-Value | Trend |
| Beta-lactams antibiotics | ||||
| Cefepime | 47 | 67 | 0.260 | ? |
| Ceftazidime | 56 | 95 | ? | |
| Ceftriaxone | 55 | 43 | <0.0001 | â??" |
| Pip-Tazo | 55 | 39 | 0.047 | â??" |
| Others antibiotics | ||||
| Amikacin | 41 | 2 | <0.0001 | â??" |
| Gentamicin | 31 | 8 | <0.0001 | â??" |
| Ciprofloxacin | 31 | 19 | 0.016 | â??" |
| Nitrourantion | 60 | 81 | 0.064 | ? |
| Imipenem | 0 | 23 | <0.0001 | ? |
| Meropenem | 3 | 5 | 0.670 | ? |
| Trimeth/Sulfa | 44 | 13 | <0.0001 | â??" |