1. Introduction
itric acid is one of the world's largest tonnages of fermentation products. It is widely used in the food beverage industries as an acidifying and flavor-enhancing agent, pharmaceutical, chemical, cosmetic and other industries for applications such as acidulation, antioxidation, flavor enhancement, preservation, plasticizer and as a synergistic agent. The worldwide demand for citric acid is met by fermentation mainly by the process involving the filamentous fungus A. niger. A number of carbon sources may be used for citric acid fermentation. For commercial reasons, the uses of molasses, sucrose or glucose syrups are favored. The use of molasses in particular is desirable because of its low cost availability.
A. niger is capable of producing very high levels of citric acid, about 90% of the theoretical yield from a carbohydrate source. For an efficient citric acid production, the growth of Aspergillus in pellet form is desirable and this can be achieved by process optimization. There is a great worldwide demand for citric acid consumption due to its low toxicity compared with other acidulants used mainly in the pharmaceutical and food industries. Global production of citric acid has now reached 1.4 million tones and there is annual growth of 3.5-4.0 % in demand/consumption. A high rate of acidogenesis in A. niger is observed only under conditions of high glycolytic metabolism and can be induced by the addition of an excess amount of sucrose or other carbohydrates which induce a high rate of glycolytic catabolism. In this production technique, which is still the major industrial route to citric acid used today, cultures of Aspergillus niger are fed on a sucrose or glucose-containing medium to produce citric acid. The source of sugar is corn steep liquor, molasses, hydrolyzed corn starch or other inexpensive sugary solutions. Bangladesh, at present, imported cent percent citric acid from foreign countries. High production depends to a great extent on the strain used and its response to the composition of the medium can show a great deal of variability. Industrial production of this chemical by fermentation using cheap raw materials is helpful in economic development of our country. Keeping in view the future requirements and also the availability of cheap raw material, efforts were made to develop the process for citric acid fermentation, based on our local resources such as molasses from sugar mills and outer portion of jackfruit. So the purpose of present study describes the feasibility of using raw and cheap materials such as molasses and outer portion of jackfruit for citric acid fermentation and to use parent strain CA16 & gamma-ray induced mutants for high citric acid yielding strain 79/20 of Aspergillus niger.
Aspergillus niger is a haploid filamentous fungi and is a very essential microorganism in the field of biology. A. niger is cultured for the industrial production of many substances. Various strains of A. niger are used in the industrial preparation of citric acid (E330) and gluconic acid (E574) and have been assessed as acceptable for daily intake by the World Health Organization. A. niger is important because of its involvement in producing citric acid as well as industrial enzymes, such as amylases, proteases, and lipases. The uses of these enzymes are essential because of its importance for transformation to food enzymes. For example, A. niger glucoamylase is used in the production of high fructose corn syrup, and pectinases are used in cider and wine clarification. Glucose oxidase is used in the design of glucose biosensors, due to its high affinity for ?-D-glucose. A variety of carbohydrate sources such as beet molasses, cane molasses, sucrose, commercial glucose, starch hydrolysates etc., have been used for citric acid production. Among these, sucrose, cane and beet molasses have been found to be the best choice (Kapoor et al., 1982).
2. II.
3. Materials and Methods
This study was done in the research laboratory of the Department of Biochemistry and Molecular Miology at Jahangirnagar University and at Institute of Food and Radiation Biology, Atomic Energy Research Establishment, Bangladesh during July 2009 and June 2010.
Parent strain Aspergillus niger CA16 and mutant strain Aspergillus niger 79/20 were first grown on agar slant medium. Each of the properly processed substrates [Molasses, jackfruit (outer potion) and mixed substrates] was hydrolyzed by 0.05 N HCl and filtered which were then used as medium for submerged fermentation. Each substrate were divided into two groups and were fermented separately, one in the presence of Prescott salt and the other in the absence. Each of the groups of the three types of media were further divided into another two subgroups and one of them was inoculated with the parent strain Aspergillus niger CA16 and the remaining one was inoculated with the mutant strain Aspergillus niger 79/20. All the flasks were then incubated for 15 days in an incubator under same conditions. Fermented media were collected on day 3, 6, 9, 12, 15 and were subjected to estimation of residual sugar, TTA value, citric acid concentration and pH determination.
4. III.
5. Chemical Reagents and Solutions
All chemicals and reagents used in this study were of analytical grade. All aqueous solutions were prepared with distilled water. Stock solution of Prescott salt (NH4NO3: 2.23g/L, K2HPO4:
1.00g/L, MgSO4.7H2O: 0.23g/L) used in the media were prepared 10 times the media concentration and diluted as its normal strength in the experiment.
6. IV.
7. Analytical Determination
At the different stages of fermentation the culture flasks were taken out of the incubator and the medium was collected onto the screw cap test tubes by pipetting and preserved at 0oC. The appropriate amount of sample was used for the estimation of total titratable acidity, citric acid and amount of residual sugar present in the medium after fermentation.
8. V. Determination of total Titratable
Acidity (TTA)
Fermented medium (0.25ml) was diluted with 20ml of distilled water and was titrated against 0.1N NaOH solution using 2 to 3 drops of phenolphthalein as indicator. The value obtained was multiplied by 4 and total titratable acidity was expressed as ml of 0.1N NaOH required to neutralize 1ml fermented medium. The titrametric analysis of fermentation of each strain gave an indication of total acidity of the medium. The medium containing high TTA value i.e. higher acid content were then analyzed spectrophotometrically.
9. VI.
10. Estimation of citric Acid from Fermentation Medium
Citric acid was estimated spectrophotometrically by the reference method of Marier and Boulet (1958). Citric acid forms a color complex of polyvinyl keto-anhydridepolymer when it reacts with acetic anhydride and pyridine which can be estimated spectrophptometrically (Auterhoff and Schwingel, 1975). Following the growth of the organism aliquots of the medium were diluted so as to have concentration in the range of 25
11. Estimation of Residual Sugar
Before inoculation and after completion of fermentation, samples were collected for initial and residual sugar estimation, respectively.
Following the fermentation, amount of residual sugar in the medium was determined by diluting the aliquots of the medium so as containing sugar concentration range of 25-200?g per ml.
Initial and residual sugar of the medium was determined spectrophotometrically by anthrone method (Morse, 1947) using anthrone as the coloring agent with sucrose as standard.
12. VIII.
13. Results
14. a) Estimation of residual sugar at different periods of citric acid fermentation
The residual sugar concentration was different in various media during citric acid fermentation by These results showed TTA value was comparatively higher in the absence of Prescott salt for all the three types of media and for each of the stain. Throughout the incubation period the TTA value was highest in case mixed fermentation medium followed by molasses and jackfruit fermentation medium. Again, fermentation by Aspergillus niger 79/20 resulted in a comparatively higher TTA value than by Aspergillus niger CA16 both in the presence and absence of Prescott salt (Figure 19).
15. c) Estimation of citric acid accumulation at different period of citric acid fermentation
Accumulation of citric acid at different incubation periods on different media followed a very similar pattern as was seen in case of TTA value. Citric acid concentration was also different on different incubation periods with various fermentation media by the parent strain Aspergillus niger CA16 strain and the mutant strain 79/20. Citric acid concentration was found to increase gradually with the increase of incubation period and maximum citric acid concentration was found on day 12 in case of each of the three media. These results showed citric acid concentration was comparatively higher in the absence of Prescott salt for all the three types of media and for each of the strain. Throughout the incubation period the citric acid concentration was highest in case mixed fermentation medium followed by molasses and jackfruit fermentation medium. Again, fermentation by Aspergillus niger 79/20 resulted in a comparatively higher citric acid accumulation than by Aspergillus niger CA16 both in the presence and absence of Prescott salt (Figure -22).
Fermentation of citric acid for commercial production is dependent on many factors like quality of strains, nutritional composition of the media, environmental conditions, deficiency of manganese and other metals, pH, temperature and dissolved oxygen tension . Usually, Aspergillus niger gives the best yield at around 25-28ºC. Increase in incubation period resulted in the increased citric acid production. A lower concentration of sugar leads to lower yield of citric acid as well as accumulation of oxalic acid (Kovats, 1960). But the use of wild type strain of Aspergillus niger is not cost effective. So, high yielding strains were searched which will give the best yield at around the room temperature. The superior strains Aspergillus niger CA16 and gamma ray induced mutants Aspergillus niger 79/20 seem to have fulfilled the requirement. Thus these strains can be conveniently exploited for the production of citric acid from cane molasses, jackfruit (outer portion) and a mixture of the two substrates.
From the findings of this study it is clearly suggested that both fermentation medium and Prescott salt have a considerable effect on the production of citric acid. Among the media used in this study, the mixed fermentation medium was found to be most suitable for citric acid production followed by molasses and jackfruit (outer portion) media. Another important finding of the present study was that Prescott salt was found to have a negative effect on the citric acid production by the either strains of Aspergillus niger. Again the gamma-ray induced mutant strain, Aspergillus niger 79/20 had a yield efficiency more than that of the parent strain Aspergillus niger CA16 and thus considered superior to the parent strain Aspergillus niger CA16. Thus as far as citric acid production is
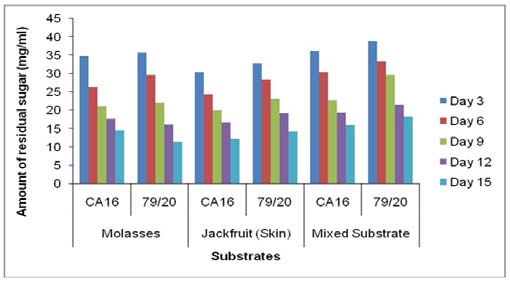
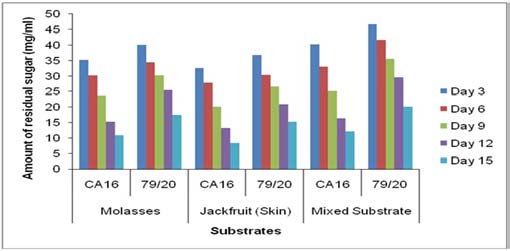
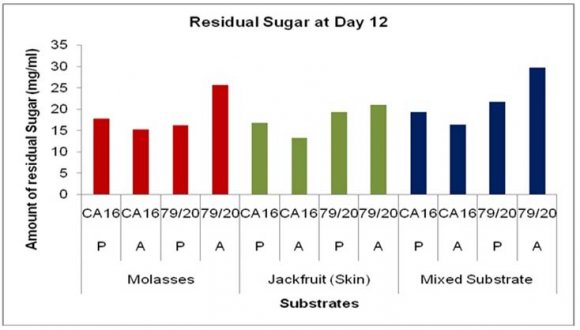
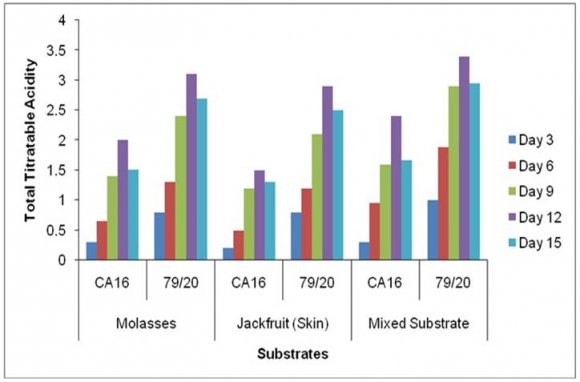
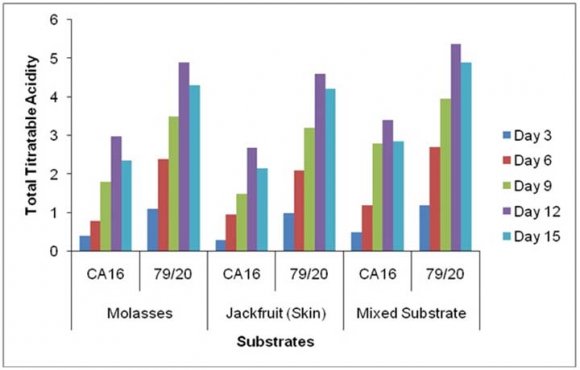
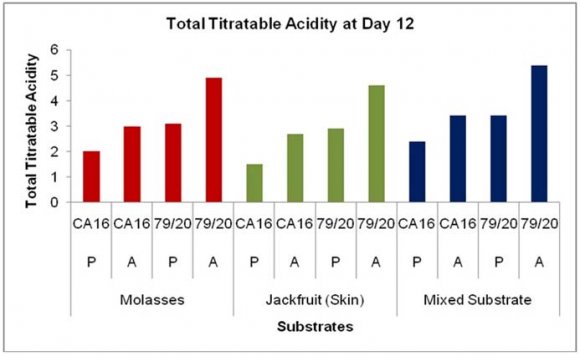
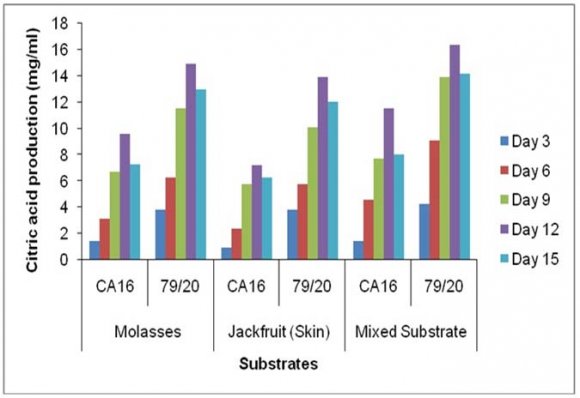
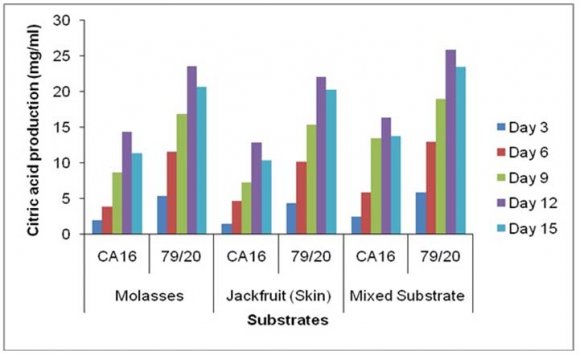
![Finally, citric acid concentration was found to decrease at day 15 [(Figure-20, 21 & 22) and (Appendix-III & VI)].](https://medicalresearchjournal.org/index.php/GJMR/article/download/1413/version/101974/4-Optimization-of-Citric-Acid_html/31733/image-10.png)
| Volume XVII Issue II Version I |
| D D D D ) |
| ( |
| Medical Research |
| Global Journal of |