1.
countries, where live attenuated vaccines are routinely used against sheep pox virus (SPV). Sheep pox virus is a member of the family Poxviridae, genus Capri poxvirus. In this study, live attenuated Sheep pox vaccines were evaluated for humoral and cellular immunity using virus neutralization index (NI), ELISA and lymphocyte proliferation assay (XTT) beside routinely titration of life attenuated virus content of vaccine in Vero cell line which gives mean satisfactory TCID50/dose (3.34) for used vaccine batches, in addition to clinical examination of vaccinated sheep and also application of challenge test. Sixty susceptible lambs were divided into (10) groups and vaccinated with field and safety doses of (10) different batches of live attenuated vaccine intradermal (I/D) in tail fold while three lambs kept as control. The results showed that lymphocyte proliferation began to increase till reach to its peak (1.312) at 10th day post vaccination then decrease after that with re-increasing after challenge , serological assays results revealed that protective serum antibody titer started at 10th day post vaccination with mean titer (1.6 and 1.99), mean absorbance (1.56 and 2.02) and at three weeks the mean titer (2.35 and 2.61) , mean absorbance (2.43 and 2.51) for NI and ELISA respectively, also all vaccinated lambs showed satisfactory levels of protection against the virulent SPV through challenge test as SID50 more than (2.5) for all batches of vaccine. (1). Sheep pox is a disease of sheep and goats characterized by pyrexia, generalized skin and internal pox lesions, and lymphadenopathy (2). Sheep pox and goat pox are ancient diseases that are currently endemic in the Middle East, the Indian subcontinent, and Central and Northern Africa. Kids and lambs are generally more susceptible than adults (3).
2. GJMR-G Classification
Vaccination has been considered to be the cheapest and sustainable means of disease control in the enzootic situation like India, Egypt and Middle East Author ?: e-mail: [email protected] (4). Prophylaxis using attenuated vaccines is the choice of control measure as the immunity is long lasting (5). Vaccines are considered among the most valuable and cost-effective tools for the control of infectious diseases. The development of safe and effective vaccines for the prevention and control of emerging and neglected infectious diseases is an international priority (6) and (7).
In endemic countries a variety of attenuated live vaccines have been used against SPV. Live attenuated vaccine protection is mediated by both cellular and humoral immunity (8) and (9). The virus neutralization test is the most specific serological test for evaluation of immunity against SPV, also the enzyme linked Immunosorbent assay (ELISA) had already been proved to have great potentiality as a quantitative serological tool in the detection of antibodies against several viral infections including the pox viruses. It had been proved that the sensitivity and specificity of ELISA are superior to those of other serological tests (10) and (11).
A significant number of veterinary vaccine potency tests for serial release are conducted using in vitro methods. For live viral vaccines, these include culture techniques to quantify microbial content as an indicator of antigenic content of the vaccine (12) and (13).
Potency testing for inactivated veterinary vaccines has traditionally used challenge testing of vaccinated animals with live microbes to determine the quantity of vaccine necessary to provide adequate protection. Inadequately protected and control animals that become infected usually develop significant clinical signs of the disease and/or die. However, in recent years, antibody quantification procedures have been developed and validated and subsequently replaced the challenge test for several vaccines (14), (15) and (16).
The global veterinary vaccine industry continues to actively pursue in vitro assays and the reduction in the use of animals for in-process antigen measurement and finished product potency testing (17), (18) and (19) The present work aims to use different immuneresponse assays for evaluation of live attenuated sheep pox vaccine as alternatives to challenge test.
3. S
II.
4. Material and Methods
5. a) Virus
Virulent sheep pox virus, Egyptian strain of sheep pox virus was obtained from the Pox Department, VSVRI Abbassia, Cairo. The virus had been previously isolated from a local outbreak (20) and was used for challenge test.
6. c) Animals
Sixty three susceptible native breed sheep 6 months old were screened using serum neutralization test and found to be free from antibodies against SPV.
7. d) Experimental Design
The experimental sheep were divided into ten groups (contain 6 animals/each) and each group divided into two subgroups as described in Table (1).Beside control group (Gp Co.) contains three animals, were kept unvaccinated as negative control. (10) batches of vaccine, beside one control group, kept unvaccinated as negative control. The animals were clinically observed daily to detect post-vaccinal reaction, and different blood samples were collected for cellular and humeral immune responses were evaluated.
3. Challenge test: was applied according to (10); 3 weeks post vaccination, all sheep groups and control group, inoculated with 0.5 ml of the virulent SPV through the intradermal route as five inoculums for each dilution of six tenfold serial diluted virus in both body sides of sheep. The challenged animals were kept in separate isolator under observation for (7) days, then exanimate for count of button shaped lesion and calculated sheep infected dose fifty (SID 50 ).
8. f) Samples
-Heparinized blood samples were collected from vaccinated and control animals before and after vaccination at different intervals (0, 3, 5, 7, 10, 14, 21 and 28 days) for application of the cellular immuneresponse assay. -Whole blood samples for separation of serum were collected also for application of the humoral immuneresponse assay at different intervals (0, 3, 5, 7, 10, 14, 21 and 28 days).
9. g) Evaluation of cellular immune response of the vaccine Batches
The cellular immunity was evaluated by application of Lymphocyte blastogenesis assay. It was carried out according to ( 21) and ( 22) using XTT cell viability assay kit (AppiChem). h) Evaluation of humoral immune response of the vaccine Batches -Serum neutralization test (SNT): It was carried out using the microtitre technique according to (23) where SP antibody titer was expressed as neutralizing index (NI) according to (24).
-Indirect ELISA: It was performed to evaluate the humoral immune response according to the method described by (25) and the results were expressed by Mean of Absorbance (Ab).
10. III.
11. Results
Table (2): Showed the titer values of life sheep pox virus of different Batches of vaccine using Vero cell line (T.C) which calculated as TCID 50 .
F 20x F 20x F 20x F 20x F 20x F 20x F 20x F 20x 1-(2016) - - - - - - + + ++ + + - - - - - - 2-(2015) - - - - + ++ + + ++ + + - - - - - - 3-(2015) - - - - - - + ++ + + - - - - - - 4-(2015) - - - - - - + + ++ + + - - - - - - 5-(2015) - - - - - - + + ++ + + - - - - - - 6-(2015) - - - - + ++ + + ++ + + - - - - - - 7-(2015) - - - - - - + ++ + + - - - - - - 8-(2015) - - - - - - + + ++ + + - - - - - - 9-(2015) - - - - - - + + ++ + + - - - - - - 10-(2015) - - - - + ++ + + ++ + + - - - - - - Controls - - - - - - -F 20x F 20x F 20x F 20x F 20x F 20x F 20x F 20x 1-(2016) - - - - - - + + - - - - - - V V 2-(2015) - - - - - - - - - - - - - - V V 3-(2015) - - - - - - - - - - - - - - V V 4-(2015) - - - - - - - - - - - - - - V V 5-(2015) - - - - - - - - - - - - - - V V 6-(2015) - - - - - - + + - - - - - - V V 7-(2015) - - - - - - - - - - - - - - V V 8-(2015) - - - - - - - - - - - - - - V V 9-(2015) - - - - - - + + - - - - - - V V 10-(2015) - - - - - - - - - - - - - - V V Controls - - - - - - -12. Discussion
Immunity to sheep pox involves both humoral and cellular responses (26). Antigens on the envelope and on the tubular elements of the virion surface stimulate protective antibodies. Even though it is the cell mediated immune response which eliminates the infection, antibodies limit the spread of the infection within the body. Neutralizing antibodies do play a significant role in the immunity as they have been shown to be an essential component of the protective immune response against sheep pox as the same was found to be absent in unvaccinated and pre-vaccinal serum samples (27) .Current evaluation of animal vaccines still focuses on the potency of final products in a batch-wise manner. All recent researches go in way to shafting from in-vivo to in-vitro for replacement the animal models, to ensure relevant quality attributes of vaccine batches by in-vitro evaluation of vaccines rather than by in-vivo potency tests on the final product (28).
For evaluating veterinary vaccines challenge studies were widely used under controlled conditions and sero-conversion studies, but the potency test in animals requires a large number of animals and involves unrelieved pain and suffering. A relevant in-vitro assay should provide a more accurate, reproducible, rapid, safe, vaccine potency test (29).
So, this study was performed for evaluation of live attenuated sheep pox vaccine by using different immuneresponse assays as alternatives to challenge test.
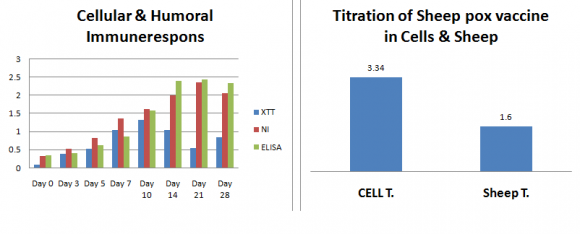
Table (2): Shows the titer values of life sheep pox virus of the ten different Batches of vaccine using Vero cell line (T.C) which calculated as (TCID 50 ) . The titer values were (? 2.5 TCID 50 / dose) for all batches in comparing with used control sheep pox virus (2.1 TCID 50 / 0.1ml) so all vaccine batches were considered Satisfactory on the level of tissue culture and these results agree with protocol of life attenuated sheep pox vaccine evaluation (30) and (31).
Table (3-1) Shows the post vaccinal body temperature changes (thermal response) of all vaccinated animals and control ones through different follow up intervals of experiment and till application of challenge test. The body temperature elevated only in sheep groups of batches (2, 6 and 10) at 5 th days post vaccination, while at 7 th and 10 th days the thermal reaction recorded in all sheep groups as the result of using the live attenuated vaccine. Also there was a mild thermal reaction for all vaccinated groups while control unvaccinated group showed severe thermal reaction post challenge due to the development of protective humoral and cellular immuneresponse of vaccinated sheep as shown in Tables (5,6 and 7) these results agree with (32).
Clinical examination of all sheep groups explained in Table G reactions appeared on the previously vaccinated animals, are due to the circulating antibodies derived through vaccination, which limits spread virus in animals (33) and (34),the results also were agreement with (35).
Table (4): Shows the titration of Vaccine Batches using Challenge Test in Sheep after being challenged with different dilutions of virulent field strain of sheep pox virus, then calculated as SID 50 and the difference between the values SID 50 of used control animal group and vaccinated groups were more than (2.5) for the vaccines and all batches considered satisfactory, these method of calculation and results were agree with (30) and (31).
It is known that sheep pox immunity depends mainly on the cell-mediated immune response in comparison to the humoral immune response (12) and (33).
The results of cell mediated immune response (XTT) expressed as the mean of absorbance in Table (5) showed the gradual increasing in lymphocyte proliferation as reached its peak on the 10 th day (1.312 and 1.415 ) then decrease to lowest level at 21 th day post vaccination (0.544 and 0.612) and re-increased to (0.827 and 0.860) post application of challenge test. These results agree with those of ( 36) and (37). Our results were in agreement with, ( 38) and ( 39) who reported the increase of lymphocyte activity by the 3 rd day post vaccination and reached its peak on the 10 th day then decreased.
Table (6 & 7) showed the results of SNT and ELISA assays. The humeral immune response increased gradually to be detected by the 10 th day post vaccination as the mean NI was (1.6 and 1.99) more than protective level (>1.5) and mean absorbance of ELISA was (1.56 and 2.02) also more than protective level (> 1) then reached to the highest level mean of NI (2.35 and 2.61) and mean absorbance of ELISA (2.43 and 2.51) at the 21 st day. These results also documented by (10) that reported neutralizing Index (NI) ?1.5 considered protective mean against Capri pox viruses and were found by ( 27) and (40) , mentioned that serum neutralizing antibodies develop on the 2 nd day and a significant rise of antibody titer was detected from the 21 st to 42 nd day post inoculation. Neutralization is very specific for almost all viruses (39). Results also harmonize with (41) and (42) who concluded that the serum neutralizing antibodies do play a significant role in the immunity against sheep pox and agree with (43) pox vaccines is the most effective immunogenic available and provide strong humoral immune response.
Table (8 & 9) and fig .
(1) Showed the collective results obtained from all methods used for evaluation of live attenuated sheep pox vaccine either in-vivo or invetro. The pattern of these results indicated the presence of co-relation between different vaccine evaluation assays with the same value and accuracy to overcome and solve the safety problems and precautions of Challenge Test. ( 14), (15) and (16).
So the positive concordance found between the antibody levels and protection in tested lambs indicates that using immuneresponse assays as method for evaluation of live attenuated sheep pox vaccine appears to be as accurate as challenge test and presents several advantages in terms of costs and speed of issue of results.
We conclude that NI and ELISA as immuneresponse assays can be reliable measure of the efficacy of vaccine batches, provided that a good correlation has been demonstrated between protective immunity and resistance to challenge in vivo. So NI and ELISA can be used as alternatives to challenge test.
| 1): Experimental Design | |||
| Groups | Batches of SHEEP POX Vaccine | Sub Groups (a) Field (b) 20X (Safety dose dose) | Number of Sheep/Gp |
| Gp CO. | CONTROL | 3 Sheep | Total # 63 Sheep |
| e) Evaluation of life attenuated sheep pox vaccine | |||
| 1. Titration of live attenuated sheep pox in Vero cell | |||
| Line by using tenfold serially dilutions of vaccine | |||
| and calculation of tissue culture infective dose fifty / | |||
| dose for each vaccinal batch (TCID 50 /dose) | |||
| 2. Potency field tests: Ten groups of sheep were | |||
| vaccinated by inoculated subcutaneously in the | |||
| ventral aspect of the tail fold with the field and (20X) | |||
| safety dose of different | |||
| Batches of | Virus Titer of | Virus Titer of | ||||||
| SHEEPPOX | Vaccine | Vaccine | Lot Dose | CONCLUSION | ||||
| Vaccine | (TCID 50 /dose) | (TCID 50 /1ml) | ||||||
| 1-(2016) | 2.5 | 3.5 | 10 dose | Satisfactory | ||||
| 2-(2015) | 2.7 | 3.7 | 10 dose | Satisfactory | ||||
| 3-(2015) | 4.5 | 6.5 | 100 dose | Satisfactory | ||||
| 4-(2015) | 4.3 | 5.3 | 10 dose | Satisfactory | ||||
| 5-(2015) | 2.5 | 3.5 | 10 dose | Satisfactory | ||||
| 6-(2015) | 4.1 | 5.1 | 10 dose | Satisfactory | ||||
| 7-(2015) | 4.3 | 6.3 | 100 dose | Satisfactory | ||||
| 8-(2015) | 3.3 | 5.3 | 100 dose | Satisfactory | ||||
| 9-(2015) | 2.7 | 3.7 | 10 dose | Satisfactory | ||||
| 10-(2015) | 2.5 | 3.5 | 10 dose | Satisfactory | ||||
| Virus Control | 2.1/0.1 ml | 4.1/1ml | --- | Control | ||||
| Days | 3-1-Thermal Response (Reaction) | |||||||
| Post Vaccination | Post Challenge | |||||||
| Batches | Day 0 | Day 3 | Day 5 | Day 7 | Day 10 | Day 14 | Day 21 | Day 28 |
| of SHEEP | ||||||||
| POX Vaccine | ||||||||
| Challenge Test in Sheep | ||||||||
| Batches of | Post Challenge | Titer of Vaccine Post Challenge | Deference Between | |
| SHEEPPOX | (I/D) Button | Test in sheep [Average of sheep | SID 50 of Control & | CONCLUSION |
| Vaccine | Shaped Lesion | infective dose 50 ] (SID50) | vaccinated Groups | |
| 1-(2016) | + | 2.3 | 3 | Satisfactory |
| 2-(2015) | + | 2.1 | 3.2 | Satisfactory |
| 3-(2015) | + | 0.5 | 4.8 | Satisfactory |
| 4-(2015) | + | 0.7 | 4.6 | Satisfactory |
| 5-(2015) | + | 2.4 | 2.9 | Satisfactory |
| 6-(2015) | + | 0.9 | 4.4 | Satisfactory |
| 7-(2015) | + | 0.6 | 4.7 | Satisfactory |
| 8-(2015) | + | 1.7 | 3.6 | Satisfactory |
| 9-(2015) | + | 2.2 | 3.1 | Satisfactory |
| 10-(2015) | + | 2.6 | 2.7 | Satisfactory |
| Mean for all Batches | + | 1.6 | 3.7 | ------ |
| Virus Control | +++ | 5.3 | control | |
| NB . Laboratory follow up of Different Batches of SHEEP POX | ||||
| Vaccine | ||||
| Table (5): Showed the results of cell mediated | ||||
| immune response (XTT) expressed as the mean of | ||||
| absorbance and clarified that the lymphocyte | ||||
| proliferation. | ||||
| Days Vaccine Batches 1-(2016) 2-(2015) 3-(2015) 4-(2015) 5-(2015) 6-(2015) 7-(2015) 8-(2015) 9-(2015) 10-(2015) Mean of means Controls Days Vacc Batches 1-(2016) 2-(2015) 3-(2015) 4-(2015) 5-(2015) 6-(2015) 7-(2015) 8-(2015) 9-(2015) | Day 0 0.075 F 0.076 20x 0.071 0.073 0.074 0.073 0.072 0.074 0.075 0.076 0.073 0.073 0.072 0.075 0.074 0.072 0.075 0.077 0.070 0.071 0.073 0.074 0.078 Day 0 F 20x 0.3 0.2 0.4 0.4 0.2 0.3 0.3 0.3 0.4 0.4 0.2 0.3 0.3 0.3 0.4 0.4 0.3 0.4 | Cell mediated immune response(XTT) (Mean of Absorbance) Post Vaccination Day 5 Day 7 Day 10 Day 14 F 20x F 20x F 20x F 20x 0.421 0.518 Day 3 20x 0.370 F 0.600 1.035 1.052 1.320 1.420 1.041 1.061 0.501 Day 21 F 20x 0.635 0.365 0.410 0.517 0.590 1.032 1.048 1.290 1.400 1.036 1.058 0.490 0.580 0.366 0.413 0.516 0.611 1.033 1.050 1.300 1.415 1.039 1.058 0.498 0.621 0.369 0.419 0.515 0.632 1.031 1.052 1.315 1.421 1.040 1.060 0.500 0.610 0.370 0.410 0.516 0.607 1.030 1.049 1.310 1.412 1.039 1.059 0.490 0.609 0.370 0.411 0.517 0.590 1.036 1.050 1.321 1.420 1.042 1.061 0.501 0.612 0.368 0.412 0.514 0.570 1.030 1.051 1.291 1.391 1.038 1.060 0.940 0.609 0.372 0.420 0.518 0.622 1.037 1.053 1.325 1.429 1.042 1.067 0.507 0.617 0.371 0.418 0.516 0.608 1.035 1.051 1.320 1.421 1.041 1.063 0.503 0.614 0.370 0.423 0.517 0.633 1.032 1.054 1.326 1.416 1.040 1.064 0.508 0.610 0.369 0.416 0.516 0.606 1.0331 1.051 1.312 1.415 1.040 1.061 0.544 0.612 0.078 0.078 0.078 0.078 0.078 0.078 Table (6): Neutralizing antibody titers Humoral immune response (mean results of Serological Examination)( NI) Post Vaccination Challenge Test in Sheep Day 3 Day 5 Day 7 Day 10 Day 14 Day 21 F 20x F 20x F 20x F 20x F 20x F 20x 0.5 0.7 0.8 1.1 1.2 1.6 1.5 1.9 1.9 2.3 2.3 2.6 0.6 0.9 0.9 1.0 1.4 1.4 1.8 2.0 2.0 2.4 2.4 2.7 0.4 0.8 0.7 1.2 1.4 1.4 1.7 1.9 1.9 2.3 2.3 2.6 0.5 0.8 0.8 1.0 1.3 1.4 1.6 1.9 1.9 2.3 2.3 2.6 0.6 0.9 0.9 1.2 1.4 1.4 1.6 1.9 1.9 2.3 2.3 2.6 0.4 0.8 0.7 1.1 1.3 1.4 1.5 2.1 2.1 2.5 2.4 2.7 0.5 0.8 0.8 1.2 1.4 1.4 1.6 2.1 2.1 2.4 2.4 2.6 0.6 0.9 0.9 1.3 1.5 1.4 1.6 2.1 2.1 2.4 2.4 2.6 0.5 0.9 0.8 1.2 1.4 1.4 1.5 2.1 2.1 2.3 2.4 2.5 Table (7): ELISA Titer * Mean of Absorbance > one considered positive (protective samples) Days Batches Of SHEEP POX Vaccine Post Challenge Day 28 F 20x 2.25 2.45 2.25 2.35 2.30 2.35 2.40 2.45 2.35 2.40 2.30 2.35 2.30 2.45 2.40 2.45 2.40 2.45 2.30 2.40 2.33 3.41 1.9 Challenge Test in Sheep Post Challenge Day 28 F 20x 0.827 2.21 3.41 0.860 1.00 1.9 1.043 2.05 2.33 Humoral immune response (mean results of Serological Examination) (ELISA) (Mean of Absorbance) Post Vaccination Challenge Test in Sheep Day 0 Day 3 Day 5 Day 7 Day 10 Day 14 Day 21 F 20x F 20x F 20x F 20x F 20x F 20x F 20x 1-(2016) 0.36 0.44 0.43 0.73 0.54 1.02 0.68 1.60 1.50 2.20 2.30 2.50 2.35 2.55 2-(2015) 0.28 0.29 0.37 0.68 0.56 0.92 0.70 1.40 1.40 1.89 2.30 2.40 2.35 2.45 3-(2015) 0.46 0.28 0.41 0.65 0.60 0.90 0.83 1.09 1.60 1.90 2.35 2.40 2.40 2.45 4-(2015) 0.37 0.45 0.45 0.75 0.64 0.98 0.90 1.20 1.60 2.22 2.45 2.50 2.50 2.55 5-(2015) 0.28 0.35 0.36 0.70 0.62 0.92 0.98 1.30 1.70 1.98 2.40 2.45 2.45 2.50 6-(2015) 0.36 0.28 0.44 0.67 0.61 0.96 0.88 1.20 1.30 1.90 2.35 2.40 2.40 2.45 7-(2015) 0.25 0.45 0.35 0.75 0.59 0.97 0.90 1.50 1.60 2.20 2.40 2.50 2.45 2.55 8-(2015) 0.43 0.30 0.42 0.65 0.66 0.90 0.94 1.30 1.70 1.92 2.45 2.50 2.50 2.55 9-(2015) 0.26 0.38 0.36 0.70 0.67 0.94 0.97 1.50 1.80 1.94 2.45 2.50 2.50 2.55 10-(2015) 0.23 0.29 0.31 0.66 0.61 0.98 0.84 1.10 1.40 2.00 2.35 2.50 2.40 2.50 Mean of means 0.33 0.35 0.39 0.64 0.61 0.95 0.86 1.32 1.56 2.02 2.38 2.47 2.43 2.51 Controls 0.35 0.33 0.35 0.34 0.44 0.45 0.40 Days Exam Assay Mean of means Post Vaccination Day 0 Day 3 Day 5 Day 7 Day 10 Day 14 Day 21 F 20x F 20x F 20x F 20x F 20x F 20x F 20x Cellular immune. (XTT). 0.073 0.074 0.369 0.416 0.516 0.606 1.033 1.051 1.312 1.415 1.040 1.061 0.544 0.612 Controls 0.078 0.078 0.078 0.078 0.078 0.078 0.078 Humoral immune. ( NI) 0.32 0.33 0.52 0.83 0.82 1.13 1.36 1.42 1.6 1.99 1.99 2.36 2.35 2.61 Controls 0.2 0.2 0.3 0.4 0.4 0.3 Humoral immune. ( ELISA) 0.33 0.35 0.39 0.64 0.61 0.95 0.86 1.32 1.56 2.02 2.38 2.47 2.43 2.51 Controls 0.35 0.33 0.35 0.34 0.44 0.45 0.40 Table (8): Collective Immuneresponse Evaluation of Different Batches of SHEEP POX Vaccine | Challenge Test in Sheep Post Challenge Post Challenge Day 28 F 20x 0.832 0.864 0.820 0.859 0.822 0.861 0.825 0.860 0.830 0.856 0.823 0.858 0.824 0.862 0.830 0.856 0.829 0.863 0.833 0.857 0.827 0.860 1.043 Day 28 F 20x 2.0 2.2 2.1 2.3 2.0 2.2 2.0 2.2 2.0 2.2 2.1 2.3 2.1 2.2 2.1 2.2 2.1 2.1 | Global Journal of Medical Research Volume XVII Issue II Version I | ||||||||||||||
| 10-(2015) | 0.4 | 0.3 | 0.6 | 0.8 Table (9): Collective virus Titeration for all vaccine Batches 0.9 1.0 1.3 1.4 1.6 1.9 1.9 2.4 | 2.3 | 2.6 | 2.0 | 2.2 | ||||||||||
| Mean of | 0.32 | 0.33 | 0.52 | 0.83 | 0.82 | 1.13 | 1.36 | 1.42 | 1.6 | 1.99 | 1.99 | 2.36 | 2.35 | 2.61 | 2.05 | 2.21 | ||
| means | Mean of | Virus Titer of Vaccine | Batches | CONCLUSION | ||||||||||||||
| Controls | 0.2 Titer for all Batches in cells (TCID 50 /dose) | 0.2 | 0.3 | 3.34 | 0.4 | 0.4 10 Batches | 0.3 | 0.4 Satisfactory | 1.00 | |||||||||
| Virus Control | 2.1 | --- | control | |||||||||||||||
| Titer for all Batches in Sheep (Challenge Test) | 1.6 | 10 Batches | Satisfactory (as deference: 5.3 -1.6 = 3.7 > 2.5 ) | |||||||||||||||
| Virus Control | 5.3 | --- | control | |||||||||||||||