1. Introduction
n the last two decades, several studies have described higher rates of operative mortality with selected surgical procedures at low-volume hospitals suggesting an inverse correlation between number of high-risk surgical procedures and mortality (1)(2)(3). The main reason claimed for explaining this inverse correlation is the lack of experience of the surgical team and, more in general, of the low-volume healthcare providers in handling complex surgical procedures associate with complex and potentially fatal complications. Although hospital volume of a few highrisk cancer procedures (e.g. pancreatectomy and esophagectomy) is a strong predictor of operative risk, relationship between volume and outcome are considerably weaker for cardiac surgical procedures, such as CABGs (4). More specifically, LaPar et al. clearly demonstrated that hospital procedure volume is not associated with in-hospital mortality for the performance of CABGs and they did not found a threshold value for hospital procedure volume at which mortality risk was significantly increased. Patient mortality risk was primarily attributable to patient-level risk factors (5).
Is it the same for patients requiring long term mechanical circulatory support (MCS)? The first study to investigate the use of long term MCS was the landmark REMATCH trial that demonstrated superior survival and quality of life in patients supported with LVAD when compared with those treated medically (52% versus 23% 1-year survival) (6). Since then, the number of hospitals accredited to perform MCS proliferated rapidly even in non-heart transplant centers. In the first 3 years after LVAD therapy approval, in the USA, the majority (53%) of 377 destination therapy (DT) recipients underwent device placement at centers that performed less than 4 DT implants (7). Lietz et al. investigated the effect of centre volume on outcomes after VAD implantation and categorized centers as small is they had implanted less than 50 devices as bridge to transplant or less than 4 as DTper year (7).
Rose et al. showed that centre experience with DT seemed to significantly correlate with the 1-year survival, but the DT centre volume was not an independent predictor of 1-year survival with DT when adjusted for the preoperative DT Risk Score, suggesting that other factors, such as improved candidate selection, may play a role in improving long term results (6). Further, a systematic review examining the influence of surgery volume on patient outcome determined that individual surgeon volume had greater effect on outcomes than institutional procedural volume (8). Therefore, the statement that institutional volume accurately represents medical expertise does not always correspond to reality.
The objective of this study is to review the outcomes of patients who were enrolled in our long term MCS program, to asses if the LVAD can be safely implanted in a low-volume, heart transplant centre.
2. II.
3. Methods
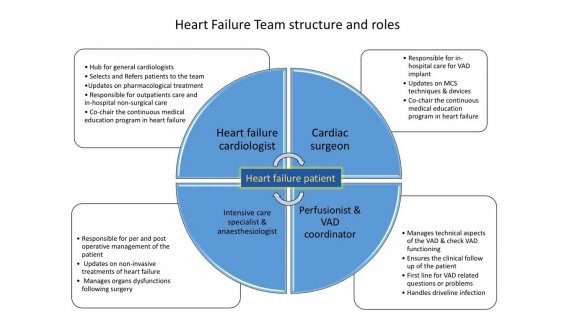
Definition of low-volume centre: centre implanting less than 50 devices as bridge to transplant or less than 4 as DT per year (7) For each patient the team defined a clear strategy of treatment including the level of therapeutic commitment in dealing with complex problems and the role of each specialists in the different phases of the therapeutic project (pre-operative, operative, postoperative and long term). Roles and competences of each member of the team are illustrated in figure 1. Data collection. Baseline clinical characteristics, pre-implant clinical course and outcomes were obtained from the medical records. The primary outcome was survival to transplant or ongoing MCS at 1year. Secondary endpoints were frequency of major adverse cardiovascular events (MACE) as defined by Kip (9). Statistical analysis. Normally distributed continuous variables were reported as mean using the Student t test. Survival analysis was performed using Kaplan-Meier method with censoring for cardiac transplantation. A p value <0.05 was considered statistically significant.
4. III.
5. Results
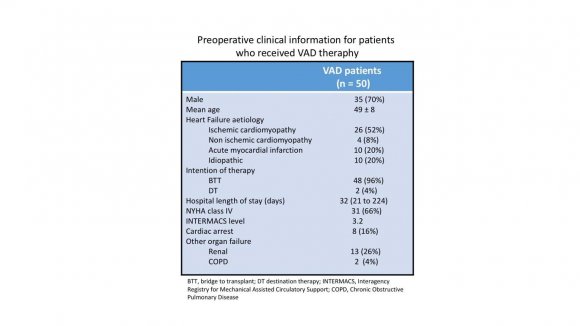
During the 10-years study period, 50 adult patients received MCS (table 1); 35 male (70%), mean age 49+/-8 years. All patients exhibited NYHA IV heart failure symptoms. Causes of heart failure included ischemic cardiomyopathy (n=26), acute myocardial infarction (n=10), idiopathic (n=10) and others (n=4). VAD was implanted as BTT in 48 and DT in 2. The devices implanted were: HeartMate II in 18 (36%), HeartWare in 20 (40%), HeartMate III in 12 (24%). Fivepatients (10%) required temporary right ventricular support with CentriMag pump (St. Jude Medical, St. Paul, MN, USA) due to failure to wean from CPB. After device implantation, antiplatelet therapy was initiated with acetylsalicylic acid and after drains removal, anticoagulation was achieved with intravenous heparin followed by transition to anti-vitamin K. From 2013 on, the Heart Failure Team handled the MCS program.
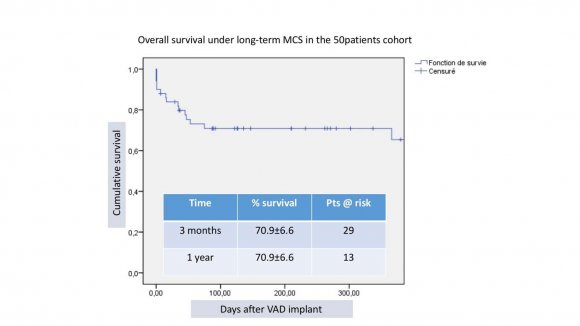
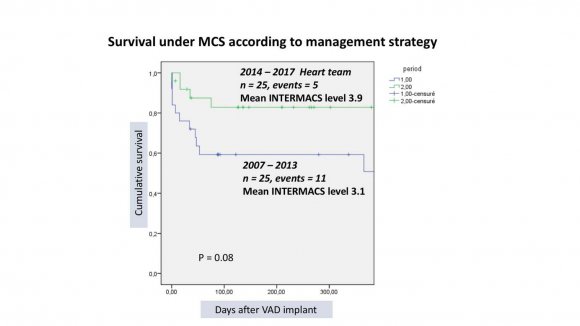
Outcomes were: death in 16 (32%),10 where in-hospital, heart transplant in 24 (48%), uneventful ongoing support 10 (20%) (figure 2). The mean waiting time under MCS before transplantation was 316 analysed according to the management team (pre and post heart team era) and 2 groups of 25 patients were identified: 2007-2013 (mean INTERMACS level 3.1) and 2014-2017 (mean INT. level 3.9) showing survival at 1 year of 56% and 80% respectively (figure 3). According to the type of device implanted, 3 groups of patients were identified: HMII = 18 (mean INT. level 2.7), HW=20 (mean INT. level 3.3) and HMIII=12 (mean INT. level 3.6), showing survival at 1 year of 52%, 78% and 91% respectively (figure 4).MACE are illustrated in table 2.
IV.
6. Discussion
The need for MCS in patients waiting for heart transplant is dramatically increasing in Switzerland (10). In the last 10 years, the number of patients waiting for hart transplant has increased of 120% while the number of tranplanted patients remains stable (in 2016, 41 received and organ and 150 were on the waiting list). We conducted this study to assess if LVAD can be safely implanted in a low-volume, heart transplant centre. At the beginning of our experience, the surgeon was the "lone star" providing VAD therapy in extremely ill heart failure patients. Patient was referred to surgeon either when in cardiogenic shock or when deteriorating on inotropes (INTERMACS profile 1 and 2). VAD implant was considered as the last "life-saving" treatment and, in such condition, the discussion on patient selection was unrealistic. Moreover, anaesthesiologists and intensive care specialists were not specifically trained to managing chronic heart failure patients with VADs. As expected, clinical results were poor encouraging cardiologists to refer patient until all other options have been exhausted. Late referrals, when patients are too sick to tolerate the LVAD surgery, further perpetuate the vicious cycle of serious operative complications, poor outcomes, and the reluctance to extend such treatment to healthier populations.The survival rate in the pre heart team era was below 60% at 1 year. We therefore decided to build a heart team dedicated to heart failure involvingalso specialists in other domains than cardiac surgery and cardiology with specific training in heart failure patients. This was independent from the number of patients treated per year that remained constantly below 20.It is well known that multidisciplinary and structured team work enhances the quality of care and we believe this is even more importantfor the management of patients requiring VAD therapy giving the complexity of the technology employed, the critically ill population and the intensive long-term postoperative medical therapy required. Chowdhury and coll., have shown that surgeon specialty training and the contribution of specialty trained members of a multidisciplinary team responsible for patient care are independently associated with improved patient outcomes (8). Our team included also anaesthesiologists, intensive care specialists, specialised nurses, perfusionistsand VAD coordinators (figure 1). Each team member received specific training in VAD therapy in high-volume centers and attending dedicated workshops and courses endorsed by the EACTS, wet-labs and meetings. They also participate to continuous medical educational program in mechanical circulatory support provided bynational and international medical associations or supported by industry. The first positive effect of the heart team approach was on patient selection. The traditional resistance to referring patients with ESHF earlier in the disease course was mitigated by directly involving the heart failure specialist cardiologists into the MCS program. Since then, the number of "crash and burn" patients reduced dramatically (from 10 to 5) and the mean INTERMACS level of the patients treated from 2014 to 2017 was higher than that of the previous group (figure 3). The surgical procedure didn't change significantly except for the number of temporary mechanical support implanted to assist the right heart.The other aspect concerns medical management in the immediate post operative phase. Intensive care specialists and cardiologists shared the experience they acquired in high volume centres on haemodynamic optimisation, including fluid and inotrope therapy, VAD settings and support of right ventricular function. Echocardiography has become an essential tool in optimising haemodynamic, identifying complications and predicting right ventricular failure (11) and all treatment adjustments are done under echocardiographic control.
The introduction of the VAD coordinator played also a key role in improving long term results that largely pertain to prevention and treatment of infectious complications, the main cause of death with DT (12). Two studies comparing early-to late-enrolment REMATCH trial (12,13) and outcomes at the 4 largest volume U.S. centers pointed to infection as the single complication, the rates of which significantly decreased as centre experience increased.The 1-year and 2-year prevalence rates of DL infection were 9% and 19% (14). Our driveline infection rate, was significantly higher thanthat reported in literature, but the clinical impact was limited to daily wound care for all patients except one who required cable transposition.
The one-year survival rate of patients treated using the multidisciplinary approach was non-inferior to the best clinical results reported in literature (15).
The MOMENTUM 3 trial has recently shown that the fully magnetically levitated centrifugal pump HeartMate 3 has a higher rate of survival free of stroke or reoperation to replace the pump at 6 months after implantation than was implantation of the mechanicalbearing axial continuous flow pump HeartMate II among patients with advanced heart failure, irrespective of their eligibility for transplantation (16). These results are consistent with the results of another centrifugal LVAD, the Heartware HVAD. In a recent report, Schmitto and al. shown excellent outcomes for patients on the device with a survival rate of almost 60% at 5 years (17). We therefore believe that the improvement in our long termresults is also due to the technical performances of new generation magnetically levitated pumps (figure 4).
Our study has several limitations. As all lowvolume centres, methodology lacks of solid scientific approach given the retrospective study design and the small sample size which limit the possibility to compare outcomes among patient subgroups. Therefore, these results may not be generalizable to other centres. It is not possible to deeply analyse statistical differences between sub-groups and clearly identify the determinants of the outcomes.
In conclusion, in this manuscript we share our experience stressing the importance of team work even if this is not supported by statistical analysis. We believe that the institutional expertise in VAD therapy have a significant impact on outcomes of this therapy, but at least in our hands, is not correlate to caseload. Long term MCS can be implanted at low-volume centres with survival rate not inferior to most recent clinical trials. Although we were not able to elucidate which aspects of centre experience were the most critical, better selection of candidates, systemic approach to surgical and postoperative care, as well as the long-term medical management, may have all contributed to the improved outcomes. Availability of a trained Heart Team with expertise in long-term MCS treatment facilitate appropriate patient selection and results improvement over time. A Heart team specifically trained in heart failure is probably more important than institutional volume in determining outcomes after VAD implantation.

| November 2007 to March 2017, either as bridge to transplant or as DT.All patients underwent heart Mechanical Circulatory Support Implanted in transplant eligibility work-up and were enrolled in the heart transplant and DT program running in our Low-Volume Centre: Heartteam Training institution. CHUV is a University Teaching Hospital | |
| where approximately 600 cardiac procedures with Counts More than Caseload extracorporeal circulation are performed annually.From | |
| 2007 to 2011, | |
| Year 2018 | |
| 7 | |
| Volume XVIII Issue I Version I | |
| I | Medical Research ( D D D D ) I |
| Global Journal of | |
| . Study design. This is a | |
| single centre, retrospective cohort study, examining | |
| clinical outcomes of consecutive patients in end stage | |
| Heart Failure who received a long-term VAD from | |
| © 2018 Global Journals |