1. I. Introduction
he accurate detection of time elapsed since death is one of the most challenges in forensic medicine. are many methods used to detect the postmortem interval, including physical, chemical and genetic methods (1).
In forensic medicine, the estimation of the time elapsed since death is important due to its role in knowing possible criminal cases and help in apply justice and penalties.
The previously known different elements of the postmortem interval can be measurable from 1 day to many years after death. The determination of the postmortem interval is based on the different changes that a cadaver passes after death including physical, chemical, metabolic processes, bacterial processes and the effect of insect activity (2). The estimating the time since death can be detected quantitatively and qualitatively (3).
Determination of the time since death by chemical means based on systematic patho-physiological alteration and are found to be more benefit since the effect of external conditions is less (4). The Concentrations of hypoxanthine, NADH, ammonia, and formic acid increased with time and these metabolites may be good markers for post-mortem interval (5). Moreover, in the post-mortem period vitreous humour Na+, K+ and Ca 2+ concentration has been investigated for many years. Various authors have found the correlation between increasing these cations and post-mortem interval (6). Additionally, the correlation between total and direct bilirubin, uric acid, urea, transferr in, immunoglobulin M (IgM), creatine kinase (CK), aspartate transaminase (AST), iron and calcium increased significantly with with the time of blood putrefaction (7).
The estimation of the time elapsed since death by molecular techniques reported that a time-dependent increase in the mRNA expression of mRNA expression of Fas Ligand (FasL) and phosphatase and tensin homologue deleted on chromosome 10 (PteN) by Quantitative-PCR. A direct linear correlation was found between the mRNA levels of both proteins up until 6 h after death, using a regression analysis (8). Additionally, postmortem serum levels of HMGB1 protein of 90 male Wistar rats preserved at 4, 14 and 24°C since death were estimated by enzyme-linked immunosorbent assay. The serum hmGB1 level showed a timedependent increase. These observation provide that HMGB1 is related to the postmortem interval in rats up to seven days at 4°C. (9).
The rationale of these study to investigate different biochemicals in serum or liver tissue homogenate enhanced identification of postmortem interval on rats.
2. II. Materials and Methods
3. a) Animal Protocol
Animal procedures were conducted with the approval of the animal care committee of the ethics Board of the faculty of veterinary medicine mansoura university (EGYPT).
25 male Albino rats (weight, 230-260 g; age were purchased from faculty of pharmacy, mansoura University, EGYPT). They were maintained on a 12-h light/dark cycle with free access to food and water. Brain stem death was induced according (10). Dead rat were kept in fixed supine position on temperature for 30 hour post mortem (august 2017).
Blood samples of each 4 rat for each time point were collected from the heart and great vessels at autopsy at 0, 2, 4, 8, 16 and 30 hours postmortem. Samples were centrifuged immediately for 20 min at 5000 rpm. Additionally, blood samples collected from living rat from retroflexus serve as control physiological group and separated for 20 min at 5000 rpm. Serum samples was stored at -70 liquid nitrogen until measurement.
4. b) Quantitative pH and biochemical activity detection
Blood pH was detected using pH meter (Sigma-Aldrich). Certified calibration buffer standards (4 or 8 pH) (Sigma-Aldrich) were used before each pH analysis. AST, ALT, BuN, creatinine and lactate dehydrogenase were analysed in serum samples.
5. c) Concentration of serum FAS ligand and TNFa were detected on by ELISA
The monoclonal antibodies of rat TNFa (sc52746) and Fas (sc-74540) used for ELISA were used as follows: ELISA plates were coated with rat TNFa and Fas antibody (5 ?g/ml) and then incubated overnight at 4°C. The plates were blocked with phosphate-buffered saline (PBS)-3% bovine serum albumin (BSA) for 1 h at room temperature and then incubated with the test sample for 4 to 5 h at room temperature. The plates were washed and sequentially incubated with biotinylated secondary antibody, avidin-alkaline phosphatase, and substrate. The OD was read at 405 nm by using ELISA reader.
6. d) Statistical Analysis
Statistical analysis was carried out using the student's t-test. P<0.05 was considered significant.
7. III. Results
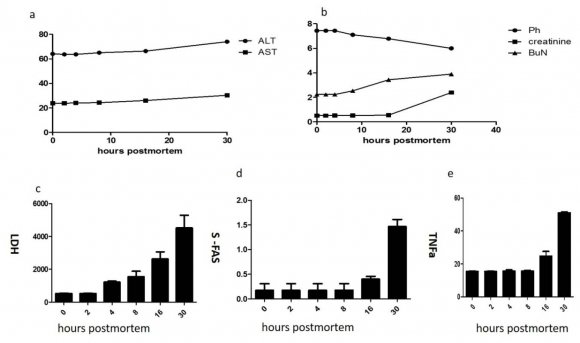
Post mortem time interval still controversial , to better understand the determinant biochemicals identify time elapsed since death, we induced death in male albino rats and measured different biochemicals in first 30 hours post death at defined times point.
AST, AlT, BUN and creatinine shown no change except little increase in last two successful time points see figure ( a, b ). Additionally, it was found that PH reduced as lactate escape from tissue while serum lactate dehydrogenase was increased in time dependent manner as leakage from tissue and may be a part of its increase due to hemolysis figure (1 c, d).
Notably, s-fas and tnfa increase in dependent manner until last time point see figure 1(d, e). On conclusion no group of biochemicals could be used in estimation of postmortem time interval for each time point. which could be explain elevation of certain biochemical parameters in serum of dead animal.
The aim of this study was to investigate metabolic changes that occur in blood post-mortem, with a view towards identifying biochemical markers that have potential for use in determining post-mortem interval. Blood pH and the concentrations of serum lactate dehydrogenase, AST, ALT, creatinine, BUN, TNFa and s-FAS changes post-mortem in rat corpses blood.
Blood is potential material as it remained liquid inside the rat corpses over the 96 hour of observation period due to release of active fibrinolysin enzymes from the vascular wall (11, 12 and 5). Post-mortem blood pH fell from 7.4 to 6.0 within the first 30 hours after death. Blood pH reduced post-mortem due to accumulation of lactic acid which resulted from anaerobic oxidation, blood stagnation in most dependant part and loss of buffer system (13 and 5).
Hepatocyte autolysis result in release of liver enzyme but this only happen when the putrefaction start in the corpse (5 and 7). While stop renal function retain creatinine but not occur immediately after death as Proteolysis did not occur uniformly throughout the body due to the resistance of some proteins such as collagen to breakdown (14). Blood does not contain substances that elevate the creatinine level Serum creatinine correlated significantly with lean mass (15) and so muscle creatinine may entered the blood and elevated the serum creatinine level.
Lactate dehydrogenase was decreased to 131 U/mg 24 hours post mortem on human dental pulp (16). Also a loss of lactate dehydrogenase activity was observed and distinguish between fresh and frozenthawed fish fillets (17). Notably, lactate and malate dehydrogenases were detected in tissue extracts of human liver kept at 5 different temperatures until 35 days after death. The investigated activities of lactate dehydrogenase were reduced in proportion to time of storage which enabled detection of time elapsed after death (18). As in the current study, serum lactate dehydrogenase activity was increased by prolonged storage of dead rat due to leakage from tissue and also little hemolysis may be a pârt of increase level of lactate dehydrogenase.
In the present study, both sfas and tnfa were increased especially at 30 hours after death induction. Increased Fas and FasL immunoreactivity was seen in the rat cortex after brain injury site from 15 minutes to 72 hours after the trauma (19). Serum sFas, TNF-? levels can be useful as biochemical markers for early selection of patients at risk of deterioration after advanced degree of traumatic brain injury (20 and 21).
On conclusion, group of biochemical could be used in estimation of postmortem time interval for each time point.