1. I. Introduction
ovine ephemeral fever (BEF) is an important arthropod-borne viral disease that affects cattle and water buffalo. It is common in tropical and subtropical regions of Africa, Asia, and Australia. It is characterized by sudden onset of fever (40.5-41°C), increase respiratory rate, nasal and ocular discharges. Muscular signs become more evident on the second day. On the third day, the animal begins eating and ruminating, and the febrile reaction disappears (Radostitis et al. 2007).
The diseases causes severe economic losses in cattle industry represented in cessation of milk production, reduction in animal condition, immobilization of animals used for draught power, impacting on trade and marketing of live animals (Walker and Klement, 2015) in addition to effect on animal reproductivity through cessation of ovarian activity and abortion (Zaher and Ahmed, 2011).
BEF was first detected in Egypt by Piot in 1895 -It was known as dengue fever of cattle-but the first detailed report about the disease was of an epizootic in 1909 (Ragbagliati, 1924). Subsequent outbreaks were recorded in 1915 and 1919-1920 It is suggested that the disease is transmitted by hematophagous insects, so the pattern of the disease is seasonal outbreaks usually occur from late spring to autumn (Walker, 2005). The insects can be transported by air current from the sub-Saharan Africa to many countries as Egypt, Israel, Syria, Iraq, Turkey, Iran, and KSA. Although some of these countries were endemic, mutation during transmission of the disease by insect might occur (Aziz-Boaron et al. 2012).
The disease is caused by BEFV which is a member of the genus Ephemerovirus, family Rhabdoviridae. BEFV has a (-ve) ss RNA genome and five structural proteins including a nucleoprotein (N), a polymerase-associated protein (P), a matrix protein (M), RNA-dependent RNA polymerase (L) and a surface glycoprotein (G) (Aziz-Boaron et al. 2012). G-protein is the target of virus neutralizing antibodies (Cybinski 1990) and its amplification for virus diagnosis (Zaher and Ahmed 2011).
Trials of diagnosis of BEF begin with the history of the outbreak and clinical exanimation of the affected animals (Walker et al. 1991). Isolation of the virus on baby mice or cell culture and confirmation of the results with IFAT are accurate and sensitive diagnostic methods of field cases of BEF (Uren et al. 1992) Although BEFV appears to have a single serotype worldwide, an analysis of some Australian and Chinese isolates has revealed some antigenic variation (Bastawecy et al. 2009). Determining the nature of these variations help in understand the epidemiology of BEFV infection and in the design of broadly protective vaccines.
As indicated by phylogenetic analysis, significant differences have been detected among the field strains circulating in the Middle East and other isolates (Aziz-Boaron et al. 2012).
The present work aimed to determine the molecular characterization of the recent isolate of BEFV with consideration of some risk factors that may affect the epidemiology of the disease.
2. II. Materials and Methods
3. a) Animals
One hundred and three Holstein Frisian cow (from total 1600 cow) reared at Al-Salhia dairy farm, Sharkia Governorate, Egypt were clinically examined according to (Rosenberger et al. 1979)
4. d) BEFV antis era
Anti-BEFV serum and locally prepared ant-BEFV serum conjugated with fluoresce in is this cyante "FITC" were supplied by DPAVR and used in VNT and direct FAT.
5. e) Baby mice
Suckling Albino Swiss baby mice (3-4 days old) supplied by DPAVR were used for trials of BEFV isolation.
6. f) Baby hamster kidney cell culture (BHK 21 )
BHK 21 cell culture was supplied by DPAVR and used for virus isolation; virus neutralization (VNT) and serum neutralization tests (SNT).
7. g) Serum neutralization test (SNT)
SNT was carried out using the microtiter technique according to (Yoneda et al. 2008) to determine the BEF antibodies in the collected serum samples and the antibody titer was expressed as the reciprocal of the final serum dilution which neutralized and inhibited the CPE of 100TCID 50 of the virus according to (Singh et al. 1967).
8. h) Virus isolation
i. In baby mice Each buffy coat and blood plasma sample was inoculated intracerebrally in each of 5 baby mice with 0.3ml/mouse according to (Hamoda et al. 2002). Inoculated mice were kept under hygienic measures in separate cages with their dams subjecting for daily clinical observations. Healthy baby mice were kept as test control. On the 3 rd to 4 th day post inoculation; when affected mice showed specific signs of BEFV infection (nervous signs; limb paralysis and cyanosis followed by death); the brains of dead mice were collected and subjected to other 2 viral passages in baby mice brains. Brain smears were prepared from affected mice and subjected to fluorescent antibody technique for virus identification.
ii. In cell culture Buffy coat samples were inoculated in BHK 21 cell culture for three successive passages according to (Azab et al. 2002) where obtained cytopathic effect was described.
9. i) Virus identification i. Virus neutralization test
Samples showing specific signs of BEFV infection in baby mice and CPE in BHK cells were subjected to VNT according to (Soad et al. 2001).
ii
10. . Direct fluorescent antibody technique (FAT)
Direct FAT was carried out on BHK 21 cell culture infected with the obtained isolates of BEFV using specific anti-BEFV conjugated with FITC.
11. j) Polymerase chain reaction (PCR)
Primers: The used primers for BEFV are demonstrated in table (1).
12. i. Nucleic acid recognition of virus samples
RT-PCR was used to amplify genome fragment from the prepared samples followed by nucleotide sequencing using BEFV specific primers. These oligos were synthesized by Bio Basic, Canada. Primers of BEF were used to amplify the expected at position 523 according to (Stram et al. 2005).
RNA Extraction was done using QIAamp Viral RNA Mini Kit (QIAGEN, Germany) Cat. No 52904 according to the manufacturer's protocol.
RT-PCR was carried out using One-Step RT-PCR Kit (Qiagen, Germany). The cycling parameters of the reaction conditions were: 95 o C for 1 min; then 35 cycles of (94 o C for 45 sec, 56 o C for 45 sec, and 72 o C for 50 sec) and then a final incubation at 4 o C overnight. Amplified products were analyzed on agarose gel. Positive and Negative control samples and DNA ladder were involved in agarose gel electrophoresis.
ii. Nucleotide sequence and Phylogenetic analysis Amplified viral RT-PCR products were sent to Macrogen Lab. (Korea) for DNA sequencing. The sequenced samples represented bovine ephemeral fever (BEFV) isolates. All the received sequence results were aligned with nucleotide sequences database at the National Centre for Biotechnology Information site (NCBI) using Basic Local Alignment Search Tool programs to assert the new sequences BEFV.
Analysis of the sequences identity phylogenetic relationship was performed using the cluster W method.
iii. Results
13. a) Clinical examination
The total number of cows at Al-Salhia dairy farm was 1600 cows. Clinical examination of these animals revealed the appearance of clinical signs leads to suspect infection with BEFV on 103 All diseased animals showed high fever more than 40 o C, salivation, respiratory distress, stiffness and some animals showed different degrees of lameness (Figure 1). The total milk production of the farm was decreased from 14,000 kg to 12,400 kg per day during the period of the diseases. According to history there were 12 cows dead along the course of the outbreak in the farm.
Disease complications appeared on six animals as tabulated in the table (2). The six cows showed different forms of recumbency including sternal and lateral recumbency (Figure 2-4). From the six cows there were two cows' revealed severe respiratory signs as much harried respiration, stretching of the neck and opening mouth (Figure 3), and two cows revealed subcutaneous emphysema (Figure 4). It was found that the morbidity rate of the disease in the farm was 6.4% while the mortality rate was 0.8% and the case fatality rate was 10.4%.
It was noticed that the clinical signs of the disease were sever in pregnant heifers than that in adult cows and no clinical signs were observed in calves.
The farm was an open system and suffering from a bad hygienic condition which helps on spreading of the vector of the disease in addition to the climatic condition when its occurred in the summer season.
14. b) Determination of the immune status of examined cattle
Serum samples obtained from diseased animals and subjected to serum neutralization test revealed low antibody titers (? 2 to 8) reflecting poor immune status in the tested animals Table (3). Partial nucleotides sequences of BEFV was obtained and gave the easiness to select from BEF viruses partial and complete sequences found on bank to align (Figure 10) and to construct the phylogenetic tree.
Phylogenetic analysis of the sequences identity revealed that the obtained recent isolate of BEFV (Zagazig-2015) is closely related as 90% to BEFV/ isolate EGY-2005 glycoprotein mRNA partial cds; BEFV/isolate TN-2004-124 glycoprotein mRNA, complete cds and BEFV/ isolate UL-1-2001 glycoprotein mRNA complete cds but as 88% with BEFV/ isolate EGY-2012 glycoprotein G(G) gene partial cds.
15. IV. Discussion
BEF is an important viral disease that causes severe economic losses in cattle herds. These losses are due to decrease milk production which usually doesn't return to the normal level at convalescence, mortality rates 1-10%, abortion in some cases, temporary loss of body weight, culling due to infertility in bulls,
The recorded clinical symptoms on affected cattle in this study including fever, harried respiration; lameness and recumbency came in agreement with those reported by (Hassan et al. 1991 ). Complication of the disease was recorded in some cases and manifested by severe respiratory signs, paralysis and subcutaneous emphysema. Similar signs were recorded by (Hassan 2000, Zaghawa et al. 2000). Cases of mastitis, abortion at the late stage of pregnancy and temporary infertility of bulls were reported by (Nandi S. and Negi, B.S. 1999).
Pathology and clinical signs related to BEFV infection are thought to be mainly attributed to increasing the vascular permeability and the cytokine storm resulting from the inflammatory response associated with the diseases (St George 1993). Pulmonary and subcutaneous emphysema may be attributed to nutritional selenium deficiency (Odiawo 1989).
In this study, the morbidity rate of the disease was 6.4% which is considered low compared with the morbidity recorded in Saudi Arabia during 1996 (59%) (Abu Elzein et al. 1997). The difference between two rats may be attributed to the previous vaccination of animals in the present study. Animals showed the clinical signs of the diseases which led to the suppression of their immunity. Also, the absence of the clinical signs of the diseases in calves in this farm may be due to coloustral antibody come from naturally infected or vaccinated dams (St. George et al. 1986).
The short incubation period, rapid onset and recovery of the BEF disease may be interpreted by the key role of released neutralizing antibodies in protection against the diseases (St. George, 1985).
Regarding the level of BEF antibodies in the examined vaccinated and affected cattle; serum neutralization test revealed that such animals had poor immune status with the antibody titer of ?2 -8 (table-3) leading them to be susceptible for virus infection. In this respect (Ting et al. 2014 Also, the infection in vaccinated cattle could be attributed to vaccination failure which may be due to the stress of pregnancy and high milk production on the general health of vaccinated cows. Also, the vaccination regime in the farm may play a significant role in the vaccination failure as the farm used the live BEFV vaccine in two doses with 28 days interval. This may consider as a load on the immune system of the vaccinated animals. (Inaba et al. 1974) recorded that vaccination with live attenuated vaccine followed by booster dose of inactivated vaccine lead to stronger immunity and more durable neutralizing antibodies response than using the live vaccine alone or two doses of inactivated vaccine.
On a contrast another side it was noticed that the used BEF vaccine might consider as a potent inducing 92.8% protection (103 infected animals and 12 dead cases from total 1600). Similar findings were obtained by (Daoud et al. 2001a andElbehwar et al. 2010) who stated that the use of live BEF vaccine induced high levels of specific BEF neutralizing antibodies.
It was successful to isolate BEF virus from the buffy coat and blood plasma in suckling mice inducing limb paralysis; cyanosis and death within 3-4 days post infection in agreement with (Habbak 2005 and Abd El-Azeim, 2008).
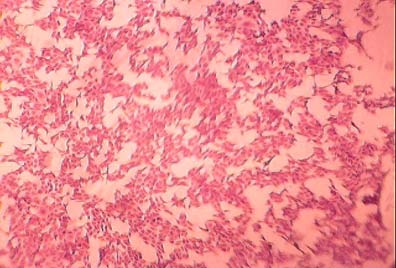
It was found that inoculation of BHK21 cell culture with the obtained virus isolate induced specific CPE of BEF which characterized by cell rounding, cell aggregation followed by detachment of the cell sheet (photo-6) in agreement with what reported that BEF virus was isolated and propagated on different cell cultures as Vero cells (Soad et al. 2001); Vero, BHK and MDBK (Azab et al. 2002 andZaghawa et al. 2006) and BHK (Zheng et al. 2011).
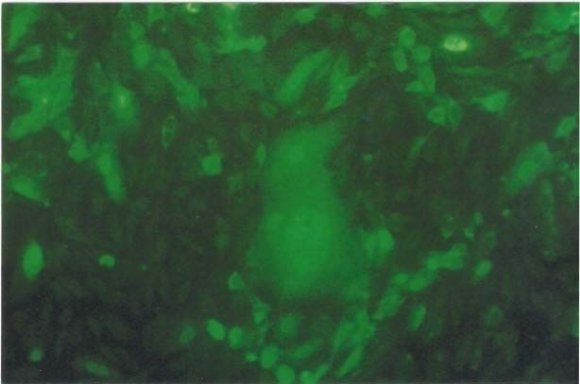
On the other side, the results of direct FAT came to confirm the presence of BEF virus showing the intracyto plastic fluorescent reaction (figure -7). The use of direct FAT for detection of BEF virus in cell culture was established by (Habbak2005 and Abd El-Azeim 2008) obtaining similar results.
Reverse transcriptase polymerase chain reaction (RT-PCR) has been developed with many advantages as it is possible to detect as little as a fragment of viral RNA from infected tissue by ethidium bromide staining after 35 cycles of PCR (Stram et al. 2005). The application of RT-PCR on isolated field virus yielded a clear single specific band on the agarose gel stained with ethidium bromide. The amplified DNA fragment corresponds to 523 BP. PCR confirms the diagnosis of BEF infection as a sensitive, specific and valuable rapid diagnosis of viral diseases.
Depending on the obtained results, it could be concluded that BEF diseases still cause a risk to the Cattle industry in spite of using a good vaccine (inducing 92.8% protection-rate under normal condition) so much attention should be paid to the risk factors of the disease.




| Sequence |
| Recorded signs | ||||
| Animal number | Recumbency | Severe resp. distress | Emphysema | |
| Sternal | Lateral | |||
| 1 | +ve | +ve | +ve | |
| 2 | +ve | +ve | ||
| 3 | +ve | +ve | ||
| 4 | +ve | |||
| 5 | +ve | |||
| 6 | +ve | |||
| Items | 0 | ? 2 | Mean serum neutralizing BEF antibody titer* 2 4 8 | 16 | Non-valid | ||
| Number of samples | 61 | 1 | 6 | 12 | 15 | 0 | 8 |
| Percentage | 64.2 | 1.05 | 6.3 | 12.6 | 15.8 | 0 | |
| Total %** | 3.8 | 0.06 | 0.38 | 0.75 | 0.94 | 0 | |
| *Mean serum neutralizing | |||||||