1. Introduction
he mycoplasmas are the smallest cell free-life microorganisms, characterized by their small cell size (0.3-0.8µm) and thus can pass through some filters used to remove bacteria. They have the smallest genome size and, as a result, lack many metabolic pathways and lack of a rigid bacterial cell wall (Mayer and Murray etal., 2002). Mycoplasmas are fastidious organisms that require specialized media for their isolation and identification (Razin and Freundt, 1984;Razinetal ., 1998). Ultrastructural studies showed that they are constructed of only three organelles: plasma membrane, ribosomes and prokaryotic chromosomes. There is no evidence of any intracellular membrane structure, and because of the lack of rigid cell wall, mycoplasmas are pleomorphic (Lin, 1985). The family Mycoplasmataceae contains two genera that infect humans: Mycoplasma and Ureaplasma, which are usually referred to collectively as mycoplasmas.Seven species of mycoplasmas can be isolated from genitourinary tract, but in female genitourinary tract the most common mycoplasmas isolated are U. urealyticum and M.hominishave been implicated in human diseases (Cassell and Cole, 1981; Krause and Taylor-Robinson, 1992). M.hominis and U.urealyticum normally inhabit the urogenital tract in sexually mature men and women. Higher isolation rates are obtained from women than from men, possibly reflecting more favorable growth conditions in the vaginal tract. The degree of colonization with these species is related to sexual activity and to the number of sexual partners (Taylor-Robinson and McCormack, 1979). In 1980 new mycoplasma species were isolated from urethral specimens from 2 of 13 men with nongonococcal urethritis. It was later named Mycoplasma genitalium because of the host tissue location, however M.genitalium strains were originally isolated from the urogenital tract. This mycoplasma shared several properties with M.pneumoniae (Tully et al., 1981(Tully et al., ,1983;;Lind et al., 1984;Taylor-Robinson, 1995).
M.genitalium and M.penetrans are recent additions to the pathogenic human mycoplasmas, reviewed by Marmion and Harris, (1996). M.penetrans. This newly-identified mycoplasma was isolated by (Lo U et alU ., 1991) from the urogenital tract of Human Immuno Virus of positive homosexual men. In a study in west Africa, M.genitalium was associated with nongonococcal urethritis (NGU), particularly in Trichomonas vaginalis negative patients (Pepin et al., 2001).
Preliminary evidence for the involvement of the mycoplasma in the cervicitis and pelvic inflammatory disease was presented by Moller et al. (1984) and Uno et al. (1997). The etiological roles of genital mycoplasmas are: Acute pyelonephritis, bacterial vaginitis, pelvic inflammatory disease, chorioamnionitis, post-abortion and postpartum fever, pneumonia in new borns, non gonococcal urethritis, prostatitis and epididymitis (Mardh and Westrom, 1970;Shepard, 1970Shepard, , 1980; Taylor-Robinson and McCormack, 1980; Krause and Taylor-Robinson, 1992;Taylor-Robinson, 1996). Furthermore, that mycoplasmas play a role in human infertility (Gnarpe and Fribreg, 1972;Stray-Pedersen et al., 1978;Casselletal., 1983;Tothetal., 1983), abortion and stillbirth (Embreeetal., 1980; Kundsin et al., 1981), premature birth and low birth weight of infant (Braun et al., 1971;Embreeetal., 1980;Kundsin et al., 1981).
Despite numerous studies of these organisms, their role in most genital infection remains controversial and illdefined. The ubiquity and low virulence of genital mycoplasmas make it difficult to evaluate their role in producing genital infections, a situation that is further complicated by their frequent isolation together with other sexually transmitted diseases (Watts and Eschenbach, 1988). Furthermore the presence of both genital mycoplasmas (U.urealyticum and M.hominis) in a large proportion of healthy women complicates the assessment of the pathogenic role of these organisms (Taylor-Robinson and Furr, 1998). However, circumstantial evidence including high frequency of isolation of mycoplasmas from females with urogenital conditions or infertility warns against minimizing the importance of these microorganisms in human disease (Kundsin and Driscoll, 1970).
Mycoplasmas were the causative agents of disease in human (Krause and Taylor-Robinson, 1992) over many years passed. Despite, numerous studies conducted in Iraq on different genera of bacteria, there have been few studies on the role of genital mycoplasmas in the infectious disease (Simhairi, 1990;Al-Bahli, 1993).
2. II.
3. Materials and Methods
4. a) Study population
The population under study was women attending the outpatient clinic of obstetric and gynecology department of Basrah General Hospital. The samples obtained consisted of pregnant women at different gestational periods and non pregnant women including women using contraceptive devices besides women not using contraceptive devices and those with infertility and those with various complaints. Also women presented with different types of abortions are included. The samples were selected randomly from the women above and collected from different parts of Basrah. A total number of two hundred and twenty two women (222) were investigated. Their age ranged from 17 to 50 years. The investigation period extended from 1 st of December, 2003 to 2 nd of October, 2004. A control group consisted of one hundred (100) healthy women.
5. b) Collection and Inoculation of samples
Two swabs one from endocervix and the other from high vagina were obtained from each woman and each was inoculated. Onto a suitable medium. A sterile speculum was used. All swabs were transported to the laboratory within (one) hour for culture. For the isolation of mycoplasmas, each specimen was directly inoculated into the liquid phase, mixed up well and tilted for a while, once or twice, to cover the upper slanted portion in a simple monophasic -diphasic culture setup (MDCS) prior to incubation (Al-Sulamietal., 2002). For the isolation of bacteria other than mycoplasmas another two swabs from endocervix and high vagina were obtained from the same women included in the study. Then, each specimen after being transported to the laboratory was directly cultured onto MacConkey & Blood agar by the streaking method then incubation follows.
6. c) Culture Media
Media for isolation and identification species of genital mycoplasmas were applied depending on Marmion and Harris [5].
7. d) Cultivation and isolation of mycoplasmas (genital mycoplasmas)
Endocervix and high vaginal swabs were taken from the women under study, then, each specimen was directly inoculated into the liquid phase of the MDCS, mixed up well and then tilted once or twice to cover the upper portion of the slant for a while prior to incubation. All inoculated media were incubated aerobically at 37C o and observed daily for colour change from red to yellow in the liquid phase after 24 hrs. Then isolated colonies appeared after that on the slanted solid phase. We added sheep erythrocytes (7%) to the solid phase of the MDCS in several trials to detect the blood haemolysis by M.fermentans and U.urealyticum. as well, the egg yolk suspension (15 ml) was added to the standard PPLO agar for detect the lipolytic ability of M.fermentans.
8. III.
9. Results
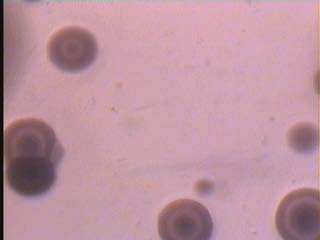
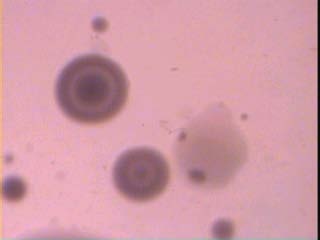
In our knowledge this study performs the first isolation of M.fermentans, M.genitalium and M.penetrans in Iraq with MDCS system and modified PPLO media according to the previous studies. Besides, it is the first isolation to both species: M.hominis and U.urealyticum by this method. The colonial growth was observed on the upper portion of the slant together with changing in colour of the liquid phase from red to yellow after 24 hrs. However, approximately 96 hrs. Period was necessary for full development of colonies on modified media of PPLO with MDCS for show the fried-egg appearance Figure 1 educational level and being frequently isolated from women with a low level (p<0.01; p<0.05; p<0.05) respectively. A difference was found to be significant in the isolation of both M.hominis and M.genitalium from non-pregnant when compared with pregnant women. A significant difference was also noted in the isolation rates of: M.hominis, M.fermentans and M.penetrans from pregnant women at different gestational periods (trimesters) as shown in Table 3, 4. A statistically significant difference at the level of (P<0.01) was noted in the isolation of both U.urealyticum and M.genitalium from endocervix region in comparison with high vaginal region. This study revealed a significant association between the five species of genital mycoplasmas and some cases or symptoms in the female genital tract such as: vaginal discharge, itching and dysuria (Table 5, 6). Also, the genital mycoplasmas were found as a single infection in 20 cases (16.9%) and mixed infection with other causative agents in 21 cases (17.5%) Table 7. IV.
10. Discussion
Prevalence of mycoplasmas in the female genital tract depends on numerous factors such as age, level of socioeconomic status, sexually active, disorders in the menstruation, pregnancy, infertility, urogenital complaints and use of contraceptive device [19-21, Reham Al-Mosawi, 2009]. According to our knowledge, this study was the third in Iraq to determine the prevalence of genital mycoplasmas in Iraqi women and the second in Basrah. Further, the current study shows for the first time the isolation and identification of: M.fermentans, M.genitalium and M.penetrans in Iraq besides the first isolation of both M.hominis and U.urealyticum by using modified PPLO medium and MDCS system for the primary isolation of genital mycoplasmas from clinical samples then, detection with biochemical test [18, Holt et a, 1994]. The modified PPLO medium consist of antimicrobial agents: such as thallium acetate and penicillin, the other supplements consist of glucose solution; sodium deoxyribonucleate (DNA calf thymus) solution; hydrogen phosphate solution and cresol red solution as an indicator of the mycoplasmal growth in addition to PPLO broth/agar media; yeast extract; sodium chloride and horse serum, this method offered multi merit symbolized by the less contamination as a result of elimination of transport media, fast of results appearance, supply a pliability in the of media represented by liquid and solid phases, it is inexpensive because its consumed a small quantities of liquid and solid media furthermore, it has a short incubation time period within one test tube. It is the first technique used to determine the prevalence of genital mycoplasmas in women in Basrah City in comparison with studies of Simhairi (1990 andAl-Bahlis' (1993).
Table 1 shows, the four species of genital mycoplasmas: M.hominis, U.urealyticum, M.genitalium and M.penetrans were more frequently isolatedin age groups 20-29 and 30-39 years whereas, M.fermentans tend to be frequently distributed in age group less than 20 years (p<0.01), this association could probably due to increase the sexual activity during this age of Iraqi women's life [21,26]. Concerning the educational levels as apparent in Table 2, the species: M.hominis, M.fermentans and M.genitalium were significantly related to the educational level and being frequently isolated from women with a low level .This is because the colonization of genital mycoplasmas in human is linked to younger age, lower socioeconomic status, and sexual activity with multiple partners and other factors [19,21]. A difference was found to be significant in the isolation of both M.hominis and M.genitalium from non-pregnant when compared with pregnant women. A significant difference was also noted in the isolation rates of: M.hominis, M.fermentans and M.penetrans from pregnant women at different gestational periods (trimesters) as shown in Table 3, 4. Simhairi (1990), Al-Bahli (1993) found no significant difference in the prevalence of M.hominis and U.urealyticum among pregnant women as compared to non pregnants, as well as, to gestational stage of pregnancy. Their results were somewhat consistent with the results of the study. Similar as well as contrary observations regarding the prevalence of M.hominis of other strains in pregnant women have been reported by many investigators (Csonka et al., 1966;Jones, 1967;Harwick et al., 1970;Delouvois et al., 1975;McCormack, 1979 andIwaska et al., 1986a). This controversy may be attributed to several factors like strain variations of the microorganisms, difficulty in determining a precise matching control with respect to sexual experience, especially in relation to the number of partners and population peculiarities, like differences in the socioeconomic status and hygienic standard . In present study we found,a statistically significant difference at the level of (P<0.01) in the isolation of both U.urealyticum and M.genitalium from endocervix region in comparison with high vaginal region(Table5), the results ofpresent study in agreements with R. M. Al-Mosawi, 2009. And in study of Upadhyaya et al. (1983) the frequency of the isolation of U.urealyticum was significantly higher in the infertile group than in a group of pregnant women. Thereupon, the results of the current study which show that both U.urealyticum and M.genitalium were recovered in a higher isolation rates from endocervix than high vagina of infected women may be associated with the inflammatory disease leading to infertility. Several investigators have demonstrated some kind of association between female infertility and U.urealyticum infection (Gnarpe and Friberg, 1973a;Fowlkers et al., 1975;Upadhyaya et al., 1983;Taylor-Robinson, 1986).
This study revealed a significant association between the five species of genital mycoplasmas and some cases or symptoms in the female genital tract such as: vaginal discharge, itching and dysuria (Table6)my study in accordance, with R. M. Al-Mosawi, 2009 except of the species M.genitalium which tend to be frequently distributed in women complaining of urethral discharge followed by vaginal discharge and itching also, Simhairi (1990) found that, the conditions like vaginal discharge, itching and monilia-associated infection were more frequent among patients with M.hominis infection than among those having no such infection. This reflects a significant degree of association between M.hominis infection and those conditions.
Similar results were reported by several investigators (Bercovici et al., 1962;Mendel et al., 1970;Taylor-Robinson and McCormack, 1979) who showed a high isolation rate of M. hominis in females with monilial and other vaginal conditions. Also, in the current study, the genital mycoplasmas were found as a single infection in 20 cases (16.9%) and mixed infection with other causative agents in 21 cases (17.5%) as showed in Table 7, these results were in agreement with R. M. Al-Mosawi, 2009.
| Volume XIX Issue I Version I |
| D D D D ) |
| ( |
| Medical Research |
| Global Journal of |
| No. and (%) of women + ve in | ||||||
| Age year | No. of tested women | M.hominis | M.fermentans | U.urealyticum | M.genitalium | M.penetrans |
| <20 | 10 | 1 (10.0) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) |
| 20 -29 | 50 | 7 (14.0) | 3 (6.0) | 6 (12.0) | 2 (4.0) | 2 (4.0) |
| 30 -39 | 40 | 6 (15.0) | 1 (2.5) | 4 (10.0) | 3 (7.5) | 1 (2.5) |
| ? 40 | 20 | 2 (10.0) | 0 (0) | 2 (10.0) | 0 (0) | 0 (0) |
| Total | 120 | 16 | 5 | 12 | 5 | 3 |
| X 2 | 1.694 | 7.361 | 11 | 13.08 | 7.192 | |
| P | NS | 0.01 | 0.01 | 0.01 | 0.01 | |
| No. and (%) of women + ve in | ||||||
| Level of education | No. of tested women | M.hominis | M.fermentans | U.urealyticum | M.genitalium | M.penetrans |
| LOW | 65 | 11 (16.9) | 4 (6.1) | 7 (10.7) | 4 (6.1) | 3 (6.4) |
| Moderate | 35 | 4 (11.4) | 1 (2.8) | 3 (8.5) | 1 (2.8) | 0 (0) |
| High | 20 | 1 (5.0) | 0 (0) | 2 (10.0) | 0 (0) | 0 (0) |
| Total | 120 | 16 | 5 | 12 | 5 | 3 |
| X 2 | 7.533 | 6.231 | 0.675 | 6.231 | 3.112 | |
| P | 0.01 | 0.05 | NS | 0.05 | NS | |
| No. and (%) of women + ve in | ||||||
| Women subjects | No. tested | M.hominis | M.fermentans | U.urealyticum | M.genitalium | M.penetrans |
| Pregnant | 40 | 3 (7.5) | 1 (2.5) | 4 (10.0) | 0 (0) | 2(5.0) |
| Non-pregnant | 50 | 8 (16.0) | 2 (4.0) | 5 (10.0) | 2 (4.0) | 1 (2.0) |
| X 2 | 12.040 | 0.346 | 0 | 4.000 | 2.25 | |
| P | 0.01 | NS | NS | 0.05 | NS | |
| No. and (%) of women + ve in | |||||||
| Pregnant women | No. tested | M.hominis | M.fermentans | U.urealyticum | M.genitalium | M.penetrans | Total of G.M (%) |
| First trimester | 10 | 1 (10.0) | 1 (10.0) | 1 (10.0) | 0 (0) | 1 (10.0) | 4 (40.0) |
| Second trimester | 10 | 0 (0) | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 1 (10.0) |
| Third trimester | 20 | 2 (10.0) | 0 (0) | 2 (10.0) | 0 (0) | 1 (5.0) | 5 (25.0) |
| X 2 | 10.110 | 23.333 | 0 | 0 | 10.000 | 34.000 | |
| P | 0.01 | 0.01 | NS | NS | 0.01 | 0.01 | |
| No. and (%) of women + ve in | |||||
| Sources of isolation | M.hominis | M.fermentans | U.urealyticum | M.genitalium | M.penetrans |
| Endocervix | 7 (43.7) | 2 (40.0) | 7 (58.3) | 3 (60.0) | 1 (33.3) |
| High vagina | 9 (56.2) | 3 (60.0) | 5 (41.6) | 2 (40.0) | 2 (66.6) |
| X 2 | 6.75 | 20.0 | 17.33 | 20.0 | 36.11 |
| P | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| No. and (%) of women + ve in | ||||||
| Uorgenital complaints | No. of tested women | M.hominis | M.fermentans | U.urealyticum | M.genitalium | M.penetrans |
| Vaginal discharge | 60 | 9 (15.0) | 4 (6.6) | 7 (11.6) | 4 (6.6) | 2 (3.3) |
| Dysuria | 20 | 2 (10.0) | 0 (0) | 1 (5.0) | 0 (0) | 0 (0) |
| Itching | 40 | 5 (12.5) | 1 (2.5) | 4 (10.0) | 1 (2.5) | 1 (2.5) |
| Total | 120 | 16 | 5 | 12 | 5 | 3 |
| X 2 | 4.23 | 8.36 | 5.66 | 4.88 | 4.25 | |
| P | 0.05 | 0.01 | 0.01 | 0.05 | 0.05 | |
| Total of genital mycoplasmas | Alone | S.aureus | In conjunction with K.pneumoniae P.aeruginosa | E.coli | Proteus. spp | |
| 41 | 20 | 7 | 5 | 4 | 3 | 2 |