1. Introduction
iabetes mellitus (DM) is a metabolic disorder whereby either the pancreas does not produce enough insulin (a hormone that regulates hyperglycemia), or situation where the body cannot effectively use the insulin it produced [1]. Correspondingly, DM has not only assumed a pandemic proportions worldwide but has also proven to affect the developing countries of the world much more than their developed counterparts [2]. Of course, DM has been demonstrated to be a prime global health concern with a projected rise in prevalence from 171 million in 2010 to 366 million in 2030 [1]. Disturbingly, both the number of cases and the prevalence of DM has steadily been showed to be on the rise over the past few decades, and it is regarded to be a silent killer disease, affecting millions of peoples in the world [3,4]. Considering Africa, studies has revealed that, the number of people with diabetes will increase from 14.2 million in 2015 to 34.2 million in 2040 with majority of the cases predominated in some of the region's most populous countries like: South Africa, the Democratic Republic of Congo, Nigeria, and Ethiopia [2,3].
Regardless of the numerous conventional medications that have been in use for the management of DM, it inaccessibility has been a limitation as a result of the relatively high cost and sometimes unavailability [5]. In this light, of course, a switch to a readily available and cheaper alternative has become necessary in the form of phyto/herbal medicine [5]. Herbal medicine similarly referred as phytomedicine; alludes to the use of plants seeds, flowers, roots for medicinal purpose and even today, plant materials continue to play an important role in primary health care as a therapeutic remedy in many developing countries [5]. As reported, the World Health Organization (WHO) recently recommended the use of medicinal plants for the management of DM and further encouraged the expansion of the frontiers for scientific evaluation on the hypoglycemic properties of diverse plant species [5,6].
Soybean-based foods have shown to be beneficial on human health and currently the consumption of soybean products elevated due to functional food improving knowledge [7,8]. Generally, it has been demonstrated that a diet high in fiber is useful in the management of the plasma glucose concentration in individuals with diabetes [9]. The beneficial metabolic effects of dietary fiber are long lasting and clinically relevant both in types 1 and type 2 diabetic patients [10]. Also, Fiber has been studied in the treatment of diabetes for many years because increased fiber content has been shown to decrease the glycemic index of foods. The theory, then, is that the decreased glycemic index would lead to smaller increases in blood glucose, and thus reduced blood glucose and HbA1c levels [11].
Okara is the insoluble residue from soybean after milk production and is mainly rich in dietary fiber 50-60% and protein 30% [12]. Its dietary fiber has 12% hemicellulose, 5.6% cellulose, 12% lignin, and 0.16% phytic acid [13]. Because of its high fiber content, okara is used as a supplement in human diets, particularly western diets, which are deficient of the essential fiber [12].Although there have been several reports on the nutraceutical power of okara [7,14,15], still, the use is not yet a common practice, as most people still dispose of it in the form of chaff after extraction. In this light, continual evaluation and research are required to validate and update the existing report on the health benefit of okara. Therefore, the aim of this study was to investigate the antidiabetic ability of okara (soybean residue) onstreptozotocin induced diabetic male Wistar rats.
2. II.
3. Material and Methods
4. a) Seed collection and preparation
Soybean Seeds were purchased from Bodija market, Ibadan, Oyo State. Thereafter, it was identified by a botanist in the Botany Department of the University of Ibadan. The seeds were sorted manually to remove defective seeds and other extraneous materials and then washed. The washed soybean seeds were blanched in hot water for 25minutes at 100 o C and then dehulled. The dehulled cotyledons were washed with hot (100 o C) water twice and wet milled using 5litres of water to 1kg of the soybean seeds. The slurry obtained was mixed and filtered through a muslin cloth to remove the milk and recover the residue called okara. The fresh okara was dried using a hot-air dryer at a temperature of 70 o C, milled and sieved through 0.25mm pore sized sieve. Okara flour was then packaged hermetically and stored for analyses and diet formulation.
5. b) Analysis of feed
We carried out a proximate content analysis for protein, fat, ash, fiber and moisture according to a standard procedure as described by [16], and mineral (calcium and phosphorus) content analyses were carried out using Atomic Absorption Spectrophotometry (AAS).
6. d) Animals used
For this study, a total of 28 male Wistar rats between the weights of 100-105g were procured from the central animal house, College of Medicine, University of Ibadan, Nigeria. The male Wistar rats were kept in well-kempt and ventilated cages, and their beddings changed every three days, and were allowed free access to clean drinking water. The rats were allowed to acclimatize for two weeks before commencement of experimentation, and all the processes involved in handling and experiment were carried out according to standard protocols approved by the animal ethics committee of the department.
7. e) Induction of Hyperglycemia with Streptozotocin
Hyperglycaemia was induced with a single dose of intraperitoneal injection of streptozotocin. 50 mg/kg of streptozotocin was dissolved in (0.1 M, pH 4.5) citrate buffer and after 72 hours; ACCU-CHEK Glucometer was used to measure blood sugar level from blood samples collected from a caudal vein of the rats. Blood sugar levels between the values of (326.50±3.56 to 327.00±4.85 mg/dl) were taken to be diabetic and the values in the range of (88.67±2.16 to 97.67±1.51 mg/dl) were taken to be normal in this study.
8. f) Measurements of food intake and body weight
Daily food intake was derived by (final feed weight -initial feed weight) on a daily basis throughout the experimentation, and body weight was taken weekly with an electronic balance.
9. g) Experimental design
Each group contained seven animals. Group 1: Normal Control fed with a normal diet.
Group 2: Negative control received 50mg/kg STZ, fed a normal diet and remained untreated.
Group 3: Positive control received 50mg/kg STZ, fed normal diet, and treated with glibenclamide 6mg/kg as used by [17].
Group 4: Received 50mg/kg STZ, fed 15% okara supplemented diet as used by [7].
10. h) Biochemical analysis
ACCU-CHEK glucometer was used to measure blood sugar level, glucose-6-phosphate dehydrogenase (G6PD) was measured by spectrophotometric method using Randoxkits, Randox kit method of enzymatic hydrolysis described by [18] was used for determination
11. i) Histopathological Studies
Small pieces of the pancreatic, liver, and kidney tissues were fixed in 10% formalin solution, followed by embedding in melted paraffin wax. Histopathological assessment and photomicrography of the prepared slides were done by using an Olympus light Microscope with attached Kodak digital camera as previously described by [1].
12. j) Statistical analysis
As previously reported in [1], data were analyzed using ANOVA (analysis of variance) and mean separation was done using Duncan multiple range test and HSD Turkey. Paired T-test was used to establish a difference in timely events. P values less than 0.05 (p<0.05) were considered significant. Data were expressed as means ± standard deviation and pictorially presented in the form of charts. All statistical analysis was done using IBM SPSS Version 22 and Microsoft Excel.
13. III.
14. Results
15. a) Feed intake
Table 2 shows the cumulative feed intake of male Wistar rats. Following two weeks of acclimatization, the weight of the feed consumed by each group was recorded on a daily basis, summed up and reported weekly as day 1, day 8, day 15, day 22, day 29, day 36 and day 43 respectively. On day 1, there was no significant difference p>0.05 in the amount of feed consumed between all the groups. However, the experimental groups (B, C, and D) intraperitoneally injected with 50mg/kg streptozotocin showed a significant increase p<0.05 in cumulative feed intake relative to the normal control at day 8, day 15 and day 22 respectively. Interestingly, the cumulative feed intake from day 29 up till day 43 for 15% okara supplemented diet fed group was significantly different p<0.05 the negative control and showed no significant difference p>0.05 as compared to the normal control.
16. b) Body weight
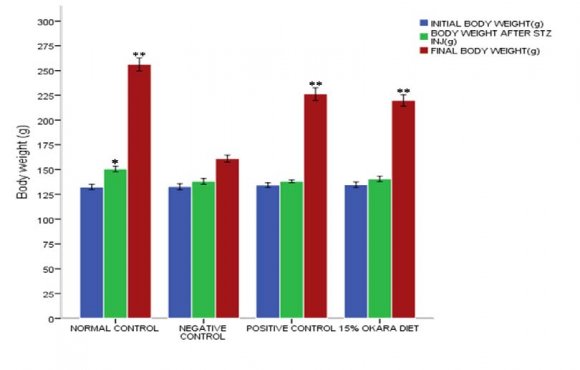
Figure 1 demonstrates the effect of 15% okara diet on body weight (g). Following two (2) weeks of acclimatization, the rats body weights were taken and reported as initial body weight, and there was no observed significant difference p>0.05 between all groups. However, after the experimental groups received 50mg/kg streptozotocin intraperitoneal injection, the body weights were recorded after 7 days, and the result revealed that there was a significant reduction p<0.05 in body weight of all experimental groups; negative control 138.17±2.86, positive control 138.17±1.33 and 15% okara supplemented diet fed group 140.50±2.81 relative to the normal control 150.67±2.73. The final body weights of the rats were recorded after treatment for a period of 43 days, 6mg/kg glibenclamide treated group 226.33±6.38 and 15% okara supplemented diet fed group 219.83±5.67 showed a significant increase p<0.05 in body weight relative to the Negative control 161.17±3.60. Similarly, the normal control also showed a significant weight increase p<0.05 compared to the negative control.
17. Medical Research
Volume XIX Issue V Version I
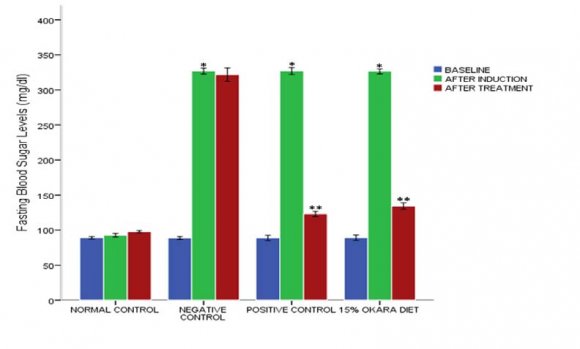
( D D D D ) F oftriglyceride, total cholesterol, and high-density lipoprotein-cholesterol. Glycated hemoglobin (HBA1c) was measured at the end of the study using a highperformance liquid chromatography (HPLC) technique. Serum level of alanine and aspartate aminotransferases (ALT and AST), alkaline phosphatase (ALP), gammaglutamyltransferase (GGT), creatinine, urea, sodium, and potassium levels were measured by spectrophotometric method using Randox kits. Superoxide dismutase (SOD) activity was measured using the method of [19] as previously described by [1], catalase (CAT) activity was measured using the method of [20] as previously described by [1], reduced glutathione (GSH) concentration was estimated using the method of [21] as previously described by [1], Glutathione-S-transferase activity was determined following the method of [22] as previously described by [1], and Glutathione peroxidase (GPX) activity was estimated following the method of [23]. Figure 2 shows the effect of 15% okara supplementation diet on blood sugar levels. Baseline blood sugar levels were recorded after two (2) weeks of acclimatization; Blood sugar levels after induction were recorded after 72 hours of intraperitoneal injection of 50mg/kg streptozotocin, and after treatment at end of 43days, blood sugar levels were also recorded. Normal control showed no significant variation p>0.05 in blood sugar levels, after treatment 97.67±1.51 relative to after induction 92.67±4.21, after induction relative to baseline 89.17±1.72. The experimental groups showed a significant elevation p<0.05 in blood sugar level after induction relative to the baseline. However, 15% okara diet supplementation and 6mg/kg glibenclamide treated group significantly lowered p<0.05 blood sugar levels after treatment relative to after induction. 3 showed that G6PD ranged from (Normal Control) 91.33±4.32 to 46.00±2.83 (Negative Control-Untreated diabetics) and HBA1c ranged from (Normal Control) 4.62±0.28 to 11.28±0.54 (Negative Control-Untreated diabetics). In each parameter assay, 15% okara supplemented diet, 6mg/kg glibenclamide (positive control) treated group, and the normal control showed a significant increase p<0.05 and a significant decrease p<0.05 relative to the negative control. Mean value with the same alphabet as superscript within each column variable are non-significant (p>0.05). The abbreviations represent G6PD: glucose-6-phosphate dehydrogenase, HBAIc: Glycated hemoglobin.
18. e) Lipid Profile
Table 4 shows the effect of 15% okara supplemented diet on lipid profile. At the end of 43 days of treatment, blood samples were collected from the rats, and plasma levels of lipid profile parameters (CHOL, TRIG, LDL, and HDL) were determined. Negative control (untreated group) showed a significant elevation p<0.05 in the levels of CHOL, TRIG, LDL, and a significant decrease in the levels of HDL relative to the Normal control. However, the treated groups (15% okara diet and positive control) significantly lowered p<0.05 CHOL, TRIG, LDL, and elevated levels of HDL relative to the untreated group (Negative control).
19. f) Liver function
At the end of the experimentation, the rats blood samples were collected, and serum levels of liver function biomarkers ALT, AST, ALP, and GGT respectively were determined as demonstrated in table 5. Negative control which had remained untreated after 50mg/kg intraperitoneal streptozotocin injection showed a significant elevation p<0.05 in all liver biomarkers assayed for relative to the normal control. However, 15% okara supplemented diet and 6mg/kg glibenclamide significantly lowered p<0.05 levels of liver biomarkers vis-à-vis negative control. Mean value with the same alphabet as superscript within each column variable are non-significant (p>0.05). The abbreviations represent ALT: Alanine Aminotransferase, AST: Aspartate Aminotransferase, ALP: Alkaline Phosphatase, GGT: Gamma-Glutamyltransferase.
20. g) Kidney Function
Table 6 shows the effect of 15% okara supplemented diet in male Wistar rats. Serum levels of kidney function markers creatinine, urea, potassium, and sodium respectively were determined at the end of the experimentation. The experimental groups received 50mg/kg streptozotocin intraperitoneal injection. However, the negative control which remained untreated showed a significant increase p<0.05 in the levels of kidney function markers relative to the normal control and treatment with 15% okara supplemented diet feeding significantly p<0.05 lowered the levels in comparison with the negative control and of course similar with the positive control. Mean value with the same alphabet as superscript within each column variable are non-significant (p>0.05).
21. h) Antioxidant Enzyme
Table 7 demonstrates the effect of 15% okara diet on selected organs of male Wistar rats. Following 43 days of experimentation, liver, kidney, and pancreas were harvested, homogenized, cold centrifuged, supernatants collected and used for determination of selected antioxidant enzyme levels. Interestingly, levels of catalase (CAT), superoxide dismutase (SOD), reduced glutathione (GSH), glutathione-s-transferase (GST), and glutathione peroxidase (GPX) for liver, kidney, and pancreas respectively were significantly decreased p<0.05 in the negative control group relative to the normal control. However, 15% okara diet supplemented fed group, and 6mg/kg glibenclamide treated group that was exposed to same levels of streptozotocin as the negative control showed a significant increase p<0.05 in the levels of selected antioxidant enzymes vis-à-vis negative control. Means with the same alphabet as superscript within each column variable are non-significantly (p>0.05). Abbreviations denote SOD: Superoxide dismutase, GSH: reduced glutathione, GST: Glutathione-S-transfer, GPX: Glutathione peroxidase.
22. Discussion
Diabetes mellitus is a serious health concern worldwide, and it is becoming the third most lethal disease of human and has continued to be on a rapid rise [24,25]. In this light, it is imperative for an unrelenting research on evaluation and validation of nutraceutical potentials of plant food for the management of diabetes mellitus. Therefore, the present study sought to carefully examine the role of Okara supplemented diet on diabetic male Wistar rats.
Polyphagia is a condition characterized by an increased appetite leading to an increase in feed/food intake. It represents the initial signs of diabetes in humans as well as in experimental models [26,7]. Owing to this finding, of course, our study demonstrated (table 2) that after diabetes induction with 50mg/kg streptozotocin from day 8 -day 15, the cumulative feed intake of the experimental groups were significantly increased p<0.05 vis-a-vis the normal control, and this may be as a result of the low levels of leptin; a critical signaling molecule in the hypothalamus influencing appetite and satiety [27]. Correspondingly, streptozotocin toxicity has been demonstrated to elicit a low level of leptin, and during uncontrolled Type 1 diabetes, plasma leptin levels rapidly fall whereas food intake increases [28,29]. However, 15% okara supplemented diets fed group from day 36 to day 43 showed no significant difference p>0.05 in cumulative feed intake relative to the normal control, and it may be attributed to the hypoglycemic potency of okara diet as seen in figure 2 and apparently, restoring the health status of the rats. Our result corroborates the finding of [7].
Streptozotocin-induced diabetes has been demonstrated to be associated with a severe reduction in body weight, perhaps, due to the degradation or loss of structural proteins that are clearly established to contribute to body weight gain [30]. Correspondingly, our study (figure 1) demonstrated that diabetes status was associated with a reduction in body weight as seen with the diabetic groups after an intraperitoneal injection of 50mg/kg streptozotocin. However, 15% okara supplemented diet feeding was significantly able to restore there rats body weight almost back to normal vis-à-vis negative control and this may be due to an improvement in diabetic status and other related abnormalities.
Diabetes mellitus is a metabolic disorder associated with an increased blood sugar levels, and of course, the result of our study figure 2, demonstrated a significant blood sugar elevation after intraperitoneal injection of 50mg/kg streptozotocin. However, 6mg/kg glibenclamide treatment and 15% okara diet supplemented diet significantly lowered the blood sugar levels, and the hypoglycemic ability of okara diet may be attributed to its high fiber content. High dietary fiber has been demonstrated to be beneficial, long lasting, and clinically relevant both in types 1 and type 2 diabetes [10] and this reports corroborate the findings of [31,14].
To further confirm the hypoglycemic ability of okara diet, glucose-6-phosphate dehydrogenase, and glycated hemoglobin levels were evaluated (table 3). Glucose-6-phosphate dehydrogenase (G6PD), an enzyme that catalyzes the first step in the hexose monophosphate (HMP) shunt an alternative pathway for the catabolism of glucose to yield pentose sugar), and Glycated hemoglobin (HbA1c) formed in a nonenzymatic pathway by hemoglobin's normal exposure to high plasma glucose levels [32]. As reported by [32], both markers are good predictors of diabetes. In this light, our study revealed that 15% okara diet supplemented diet restored the levels of G6PD and HbA1c vis-à-vis the negative control. Therefore, okara dietis a potent dietary agent for the management of diabetes.
Diabetic dyslipidemia shows high levels of plasma cholesterol (CHOL), triglyceride (TRIG), LDLcholesterol (LDL), and low HDL-cholesterol (HDL) concentrations [33]. Similarly, alterations in lipid metabolism can cause lipotoxicity, which can further exacerbate diabetic complications [34]. Also, a high level of serum triglycerides, and a low level of High-Density Lipoprotein (HDL) are listed among the constellation for the medical conditions related to metabolic syndrome [35]. Of course, the result of the present study (table 4) revealed that the diabetic group (negative control) significantly p<0.05 had an elevated level of CHOL, TRIG, LDL and a reduced level of HDL relative to the normal control. Conversely, 15% okara supplemented diet feeding significantly normalized the lipid triad and hence may have also improved the diabetic condition of the rats. Additionally, our result corroborates the finding of [13,14,36].
Markers used to determine toxic effects of administered foreign substances to experimental animals are enzymes activities. Liver function enzyme ALP, is a membrane-bound enzyme meanwhile, ALT, and AST are cytosolic enzymes [37]. Therefore, high levels of ALP, ALT and AST respectively in the serum, are indicators of cell membrane permeability and a significant degree of damage to the liver [37]. Streptozotocin, a diabetogenic agent is associated with some degree of liver damage by several studies [38,39]. In this light, of course, our result presented in Table 5 demonstrated that the levels of liver function biomarkers were significantly elevated in negative control which was exposed to 50mg/kg intraperitoneal injection of STZ relative to normal control. However, 15% okara diet supplementation potentiated a significant reduction in liver function biomarkers and this result suggests that some components of okara, namely; dietary fiber, dietary bioflavonoids, and isoflavones, could be associated with its ability in maintaining liver function.
Furthermore, one of the huge concerns of DM is its related complications, which can affect multiple vital organ systems [40,41]. Creatinine, Urea, and electrolyte are selected indices of kidney function [42]. In this light, of course, the result of the present study table 6, underscored the favorable role of 15% okara diet supplementation in abrogating renal dysfunction induced by exposure to streptozotocin, as the was a significant reduction p<0.05 in markers of kidney function vis-à-vis the negative control.
Antioxidants are key players for the investigation of oxidant stress-related diabetic pathologies, and the activities of the antioxidant enzymes catalase, superoxide dismutase, and glutathione peroxidase has been demonstrated to be reduced in diabetic conditions [43]. Correspondingly, DM is associated with an increased free radicals formation, and a decreased antioxidant capacity, consequently resulting to oxidative stress and a damage of cell components [44].
The result of the present study table 7, demonstrated that the negative control which remained untreated after 50mg/kg intraperitoneal injection of streptozotocin showed a significant decrease p<0.05 in levels of antioxidant enzymes CAT, SOD, GSH, GST, and GPX relative to the normal control for pancreas, liver and kidney respectively. The result is in tandem with the reports that kidney antioxidant enzyme activity declines in STZ induced animals as a consequence of the oxidative stress elicited by STZ [45]. Oxidative stress induced by streptozotocin (STZ) results to pancreatic beta cell damage [46], and a decline in the levels of liver antioxidant enzyme [47]. Interestingly, 15% okara supplemented diet caused a significant increase p<0.05 in antioxidant enzyme levels vis-à-vis negative control for all organs and this activity may be as a result of its composition which corresponds with the report of [48]; reported that, Okara contains phenolic compounds (106.7 mg gallic acid equivalents (GAE)/100 g) and flavonoids (32.7 mg quercetin equivalents/100 g) and showed antioxidant activity.
The histological observation of the pancreas figure A to D as contained in plate I for this study, revealed that the untreated group after 50mg/kg STZ injection (Negative control) had a severe a peritubular cellular infiltration. Perhaps, as a result of STZ diabetogenic action, by a direct irreversible damage to the pancreatic beta cells, this leads to degranulation, and loss of capacity to secrete insulin [49]. Correspondingly, the prevalence of diabetes mellitus is positively correlated with fatty infiltration of the pancreas [50]. However, the pancreatic tissue of the 15% okara supplemented diet fed group showed no visible lesions, and this highlights it ameliorative potency in the management of diabetes mellitus. The histological observation of the liver figure E to H, plate II shows that negative control (F) had random foci of single-cell hepatocellular necrosis, but this was reversed by 15% okara supplemented diet (H) as the was no visible lesion observed. However, the histological observation of the kidney figure I to L, plate III showed no visible lesion for all groups contrary the findings of [45] that histological observation of the kidney exposed to STZ was necrotic.
V.
23. Conclusion
A poor dietary habit has been demonstrated to be one of the key players in the development of diabetes mellitus, and a diet rich in dietary fiber has been highlighted to be a potent candidate for the management of DM. Thus, the result of our study shows that Okara diet; perhaps an excellent nutraceutical aids weight reduction, lower blood sugar and glycated hemoglobin levels and maintains a healthy level of lipid profile, liver, kidney, and antioxidant enzyme biomarkers. Conclusively, we recommend a supplementation of food with Okara.
| Parameters | Normal Diet (%) Okara Diet (%) | |
| Protein | 21 | 24.5 |
| Ash | 1.3 | 4.2 |
| Moisture | 4.6 | 7.4 |
| Fat | 3.5 | 3.2 |
| Fibre | 6.0 | 40.8 |
| Calcium | 0.8 | 0.9 |
| Phosphorus | 0.8 | 0.72 |
| Okara | - | 15 |
| Dry Matter | - | 91.38% |
| G | Day 1 | Day 8 | Day 15 | Day 22 | Day 29 | Day 36 | Day 43 |
| 24.8±1.17 a | 33.7±1.03 a | 50.3±1.63 a | 62.7±5.61 a | 96.3±5.28 a | 132.3±1.51 c | 174.8±1.72 c | |
| 25.0±1.41 a | 42.2±0.75 b | 71.3±1.63 b | 93.3±2.07 b | 105.7±0.82 b | 119.2±2.23 a | 156.2±1.17 a | |
| 24.5±1.64 a | 42.8±1.47 bc | 72.8±2.04 b | 92.8±2.56 b | 117.5±1.52 c | 128.7±2.42 b | 165.0±2.10 b | |
| 24.7±1.21 a | 44.2±1.47 c | 73.5±1.87 b | 93.3±1.63 b | 125.7±1.63 d | 131.5±1.52 bc | 173.3±3.27 |
| GROUPS | G6PD | HBAIc % |
| Normal Control | 91.33±4.32 d | 4.62±0.28 a |
| Negative Control | 46.00±2.83 a | 11.28±0.54 d |
| Positive Control | 74.50±5.21 c | 6.37±0.36 b |
| 15% Okara Diet | 58.17±2.56 b | 7.57±0.52 c |
| Groups | CHOL | TRIG | LDL | HDL |
| Normal Control | 51.00±1.55 a | 34.83±2.23 a | 17.00±1.67 a | 40.00±3.74 c |
| Negative Control | 70.17±1.17 c | 51.67±1.86 d | 28.67±1.63 c | 21.83±1.17 a |
| Positive Control | 57.50±3.39 b | 41.00±1.41 b | 20.50±1.87 b | 30.17±1.60 b |
| 15% Okara Diet | 56.17±1.17 b | 44.67±1.75 c | 22.17±2.48 b | 30.17±2.14 |
| Groups | ALT(U/I) | AST(U/I) | ALP(U/I) | GGT(U/I) |
| Normal Control | 32.33±2.94 a | 39.50±2.34 a | 125.67±2.94 a | 5.67±1.21 a |
| Negative Control | 44.67±2.01 b | 53.67±2.34 b | 164.17±2.86 b | 15.67±1.63 b |
| Positive Control | 58.83±2.23 c | 63.67±2.94 c | 143.25±2.51 c | 9.00±0.89 c |
| 15% Okara diet | 44.00±2.53 b | 51.67±1.86 b | 144.33±4.80 b | 8.17±0.98 b |
| Groups | Creatinine | Urea | Potassium | Sodium |
| Normal Control | 51.67±1.03 a | 15.17±1.17 a | 6.50±0.55 a | 147.50±2.17 a |
| Negative Control | 70.00±1.41 c | 26.67±1.37 b | 10.67±1.21 c | 164.17±3.00 c |
| Positive Control | 53.00±0.89 a | 18.00±1.72 a | 8.83±1.72 b | 152.67±1.63 b |
| 15% Okara diet | 55.50±1.05 b | 17.00±2.83 a | 8.00±0.89 ab | 153.00±2.10 b |
| CATALASE | SOD | GSH | GST | GPX | |
| GROUPS | LIVER | ||||
| Normal Control | 39.60±1.14 c | 43.60±2.30 d | 83.20±1.92 d | 38.40±1.14 d | 33.00±1.58 c |
| Negative Control | 15.00±1.58 a | 22.00±1.58 a | 39.00±1.58 a | 11.20±1.92 a | 12.80±1.48 a |
| Positive Control | 26.20±1.92 b | 35.60±1.14 c | 62.00±3.58 c | 26.40±2.30 c | 25.00±1.87 b |
| 15% Okara Diet | 24.20±2.77 b | 30.10±1.14 b | 56.40±3.36 b | 21.40±2.30 b | 22.20±1.92 b |
| KIDNEY | |||||
| Normal Control | 33.60±1.14 d | 35.40±2.30 c | 64.80±3.11 d | 33.40±2.70 c | 32.20±1.92 c |
| Negative Control | 10.20±1.92 a | 16.80±2.28 a | 33.00±3.16 a | 18.20±4.76 a | 11.00±1.87 a |
| Positive Control | 25.20±1.30 c | 27.80±1.64 b | 47.20±1.48 c | 24.20±0.84 b | 24.60±2.24 b |
| 15% Okara Diet | 21.00±1.58 b | 25.20±2.39 b | 42.00±1.58 b | 23.80±2.25 b | 23.40±3.05 b |
| PANCREAS | |||||
| Normal Control | 30.20±0.84 c | 32.60±1.14 c | 54.40±2.97 c | 34.80±1.30 c | 35.00±2.74 c |
| Negative Control | 11.40±1.14 a | 12.60±3.05 a | 23.80±2.39 a | 12.80±1.92 a | 11.60±1.82 a |
| Positive Control | 23.80±1.30 b | 23.20±1.79 b | 45.20±2.86 b | 25.60±2.30 b | 23.20±2.86 b |
| 15% Okara Diet | 23.40±1.82 b | 24.40±2.41 b | 43.00±2.65 b | 23.00±2.55 b | 22.60±2.61 b |