1.
Introduction peptic ulcer disease is major diseases of the gastrointestinal tract seen throughout the world 1 . The formation of peptic ulcer diseases depends on the presence of acid and peptic activity in gastric juice with a breakdown in mucosal defenses of the gastrointestinal tract 2 . The prevalence of Helicobacter pylori infection and widespread use of acetylsalicylic acid and other nonsteroidal anti-inflammatory drugs (NSAIDs) are known etiologic agents disrupting mucosal resistance to injury 3,4 . Several other pathogenic elements postulated for gastrointestinal lesions, include prostaglandins deficiency, bile acids, bacterial flora, and nitric oxide 5,6 , yet the precise mechanisms remain unknown.
Currently, the prevention and cure of peptic ulcer diseases are among global health challenges confronting medicine 2 . In our review, most reported studies give the general idea of peptic ulcer and its management using synthetic drugs demonstrated intermittent relapses and adverse drug interactions 7,8,9 .
However, many medicinal plants have been reported to possess beneficial effects in gastrointestinal disorders, especially ulcerative peptic diseases with a high level of safety compared to most synthetic drugs 10 . In developing countries, most people still rely on medicinal plants to meet their health needs, especially in cases where synthetic medicines could not provide relief from hard-to-cure illnesses 11,12 . Cadaba farinosa belongs to the capparidaceae (capparaceae) family 13 . The plant is enriched with abundant phytochemicals, including flavonoids and alkaloids are widely used in traditional medicine as antibacterial, antiprotozoal and anthelminthic agents to treats diarrheal, dysentery, and gastrointestinal parasites 13,14 . The stem bark of Cadaba farinosa served as aperients, purgative, and stomachic stimulants. In that desert of India and Pakistan, its extract is externally applied to fresh wounds to prevent sepsis, thereby assisting in healing 15,16 . In Nigeria, the analgesic and anti-inflammatory properties of Cadaba farinosa was reported among the people of Maiduguri, Jimeta, and Nguru 17 .The plant was also used in the management of gastric and duodenal ulcers by inhibition of carbonic anhydrase 18 .Hence, these findings prompted us to study the possible histological effects of the aqueous stem bark extract on the gastrointestinal tract.
2. II.
3. Materials and Method a) Experimental Animals
Wistar rats were procured from the Animal House, Faculty of Pharmaceutical Science, Usmanu Danfodiyo University Sokoto, and maintained with free access to standard animal pellets and water. The permission and approval for animal studies with Reg. NO: PTAC/Cf/OT/004-18 was obtained from the Faculty of Pharmaceutical Sciences, Animal Ethics Committee, Usmanu Danfodiyo University Sokoto.
4. b) Plant Collection
The fresh stem bark of Cadaba farinosa was harvested from its natural habitat at the Faculty of Pharmaceutical Science, Usmanu Danfodiyo University Sokoto. The plant was authenticated and deposited at the Department of Pharmacognosy and Ethno medicine, Usmanu Danfodiyo University Sokoto, Nigeria.
5. c) Plant Extraction
The fresh inner stems bark was shadow dried and pounded into small pieces using pestle and mortar. About 210g powdered plant material was soaked and extracted in 600mL of water at room temperature for 24 hours 19 . The liquid filtrates were concentrated and evaporated to dryness at 45°C in a water bath. The aqueous extract was stored at -4°C until used.
6. d) Experimental Design
The acute toxicity and LD 50 determination were carried out using Lorke's method 20 . According to guideline 423 of the Organization for Economic Cooperation and Development (OECD), the first phase consists of nine Wistar rats that were separated into three groups of three rats each and the aqueous extract was administered by gavage at dose levels 10, 100, and 1000 mg/kg/day. A cage side observation was done to detect any behavioral signs of toxicity salivation, erection of the hair, diarrhoea or mortality.
Following the absence of toxicity sign, the second phase according to Lorke's consisted of three rats that were administered with dose levels 1600, 2900, and 5000mg/kg/24 hours. The animals were observed for signs of toxicity.
In the sub-acute study, thirty (30) male and female Wistar rats were selected and randomized into five groups of six rats per group. Group 1 served as the control while the rats in groups 2, 3, and 4 were administered with plant extract by gavage at dose levels 100, 200, 300, and 400mg/kg for twenty-eight days.
7. e) Tissue Histology
The intestines of Wistar rats were excised by abdominal incision and the tissues were fixed in 10% formal saline for 24 hours before being processed and embedded in paraffin wax. The tissues were sectioned with a rotary microtome at 5µm, and the cut sections were stained with Haematoxylin and Eosin (H&E) stain 21 . The stained slides were carefully examined under a light microscope at high power magnification, and photomicrographs were taken 22 .
8. III.
9. Results
10. a) Acute toxicity and LD 50 determination
The toxicity study and LD 50 determination result (Table 2) showed that oral administration of the aqueous stem bark extract of Cadaba farinosa at dose levels 10, 100, 1000mg/kg/24hours produced no behavioral sign of toxicity or mortality.
In Phase II, oral administration of the extract at dose levels 1600, 2900, and 5000mg/kg/day, indicated neither behavioral change nor death. The animals were as active as control. Therefore, the median lethal dose (LD 50 ) of aqueous stem bark extract of Cadaba farinosa is above 5000mg/kg. IV.
11. Discussion
Gastrointestinal lesions (ulcerative peptic diseases) is associated with several pathogenic elements, including prostaglandins deficiency, bile acids, bacterial flora, and nitric oxide, 5,6 yet the precise mechanisms remain unknown 23 . However, the overwhelming proportions of chemical agents (84.45%) used in most pharmaceutical industries for the production of conventional drugs used for the management of gastrointestinal lesions are gotten from plants 24 . In the present study, the acute toxicity study revealed that oral administration of Cadaba farinosa extract up to 5000mg/kg produced no immediate signs of toxicity or mortality indicating that the LD 50 was above 5000 mg/kg, therefore, this explains that aqueous extract of Cadaba farinosa could be administered to animals with some degree of safety, through oral route where absorption might not be complete due to inherent factors limiting gastrointestinal tract absorption 25 .
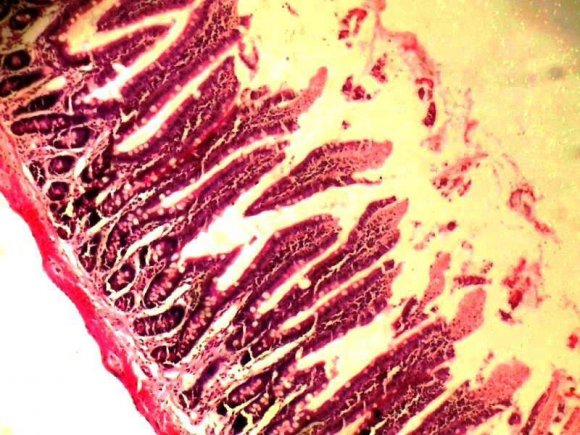
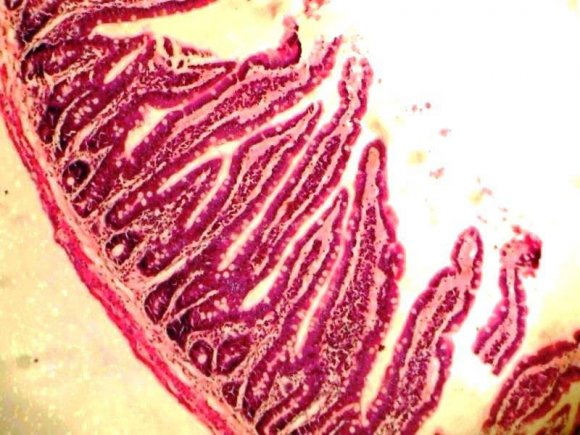
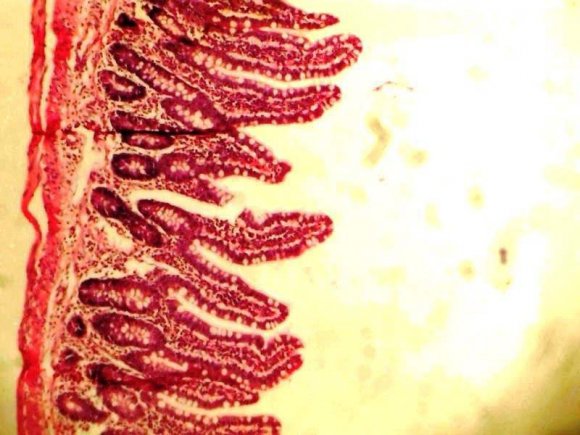
Our histological finding, showed a mucosa layer with few intestinal goblet cells lined by epithelial cells with intact submucosa and smooth muscle layers (Plate 1), while extract dose level 100mg/kg/28days showed considerably increased intestinal goblet cells and well-preserved submucosa and smooth muscle layers (Plate 2). A mucosa layer with numerous intestinal goblet cells that was lined by epithelial cells and wellpreserved submucosa, and smooth muscle layers were seen in animals at dose levels 200, 300, and 400mg/kg compared to the normal control with few intestinal goblet cells.
Intestinal goblet cells are unicellular glands 26 . These cells synthesized and secretes mucin and cyclooxygenase (COX) responsible for the synthesis of prostaglandin that is expressed in cyclooxygenase pathway 1 (COX-1) and the inducible cyclooxygenase pathway 2 (COX-2) isoforms 27 . The intestinal goblet cells following oral administration of aqueous stem bark of Cadaba farinosa is indicative of high secretion of mucin COX-2 that would preserve vulnerable cellular compartment of the gastrointestinal tract. The luminal prostaglandin modulates acid concentration by inhibiting acid secretion, alter blood flow, and stimulate mucus and bicarbonate secretion leading to dramatic protection against mucosal damage. Our result is similar to the role of endogenous prostaglandins in gastric secretion and mucosa defence 28 , the anti-inflammatory effects of prostaglandins in ameliorating mucosal damage 26,27 , and stimulation of duodenal bicarbonate and mucus secretion mediated intestinal mucosal protection 29,30,31 . These physiological functions of mucin COX-2 could modulate major etiologic factors implicated in ulcerative peptic diseases, including lesions caused by NSAIDs were effectively prevented by supplementation of exogenous prostaglandinendoperoxide synthase (PGE2) 31,32,33 .
Therefore, stem bark extract of Cadaba farinosa is a possible source of a drug, which modulates secretions of mucus, acid, and bicarbonates preceding dramatic protection against mucosal damage risk factors of ulcerative peptic diseases and gastrointestinal hemorrhage.
V.
12. Conclusion
This study showed that acute oral administration of aqueous stem bark extract of Cadaba farinosa forsk is safe up to 5000mg/kg body weight/day. Sub-chronic oral administration of aqueous stem bark extract of Cadaba farinosa forsk at the tested doses showed numerous intestinal goblet cells that secretes mucin and cyclooxygenase (COX) responsible for the synthesis of prostaglandin. Hence, Cadaba farinosa forsk is a possible source of anti-peptic ulcer drug since prostaglandins plays a critical role in the background of gastrointestinal lesions.


| Dose | Mortality | |
| mg/kg body weight | Phase I | Phase II |
| 10 | - | |
| 100 | - | |
| 1000 | - | |
| 1600 | - | |
| 2900 | - | |
| 5000 | - |
| Dose | Mortality | |
| mg/kg body weight | Phase I | Phase II |
| 10 | 0/3 | - |
| 100 | 0/3 | - |
| 1000 | 0/3 | - |
| 1600 | - | 0/1 |
| 2900 | - | 0/1 |
| 5000 | - | 0/1 |
| b) Tissue effects of plant extract | ||
| Our histological finding showed normal | ||
| intestinal goblet cells lined by epithelial cells and intact | ||
| submucosa and smooth muscle layers (Plate 1). | ||
| Extract administration of 100mg/kg/28days | ||
| showed considerably increased intestinal goblet cells | ||
| lined by epithelial cells and well-preserved submucosa | ||
| and smooth muscle layers (Plate 2). | ||