1. Introduction
ngiogenesis is a process that results in the proliferation of new blood vessels and plays an important role in growth, progression, and metastasis of the tumor. The vascular endothelial growth factor (VEGF) promotes the development of angiogenesis and over expression of the VEGF that is related to poor prognosis in numerous malignancies (1,2). This process mainly occurs by vascular endothelial growth factor signaling pathway that includes two main target components that are VEGF ligands and VEGF receptors (VEGFRs). Bevacizumab, a humanized monoclonal antibody against VEGF, has shown benefit in the treatment of patients with various malignancies such as metastatic colorectal cancer, non-small-cell lung carcinoma, by many phase III studies. There is much favorable evidence of the benefits of phase II clinical trials in patients with pancreatic cancer, renal cell cancer, and prostatic adenocarcinoma. Though bevacizumab is usually well-tolerated, it may be related to symptomatic side effects like delayed wound healing, hemorrhage, leukoencephalopathy, neutropenia, proteinuria, and nephrotic syndrome, gastrointestinal perforation and congestive heart failure.(3) Bevacizumab conjointly contributes to the event of arterial and venous thromboembolism, a typical complication resulting in morbidity and mortality in patients with malignancy. (4) We hypothesized that sample sizes in randomized control trials were not powered and large to reveal significantly increased risk. Hence we performed a Meta-analysis of published phase 2 and 3 randomized clinical trials of bevacizumab to determine the risk of arterial and venous thromboembolic events.
2. II.
3. Methodology a) Data Source
We carried out a systematic search of existing databases and after careful scrutiny by two independent researchers; Fourteen studies were selected for inclusion in the analysis. The search was done based on the preferred reporting system for meta-analysis (PRISMA) guidelines. 5 An independent review of citations from scientific databases like clinical trials.gov, Pub med central, NCBI, NIH, Cochrane Library, and Google scholar from January 2004 to January 2015 was conducted. Keywords, bevacizumab, Avastin, cancer, human studies, and clinical trial, Arterial Thromboembolic Events (ATE), Venous Thromboembolic Events (VTE) were included in the search. The search was limited only to the articles published in the English language.
4. b) Data extraction and clinical end points
All study-related Randomized controlled trials (RCTs) using either: A proper method of allocation concealment (e.g., sealed opaque envelopes), Studies that were double-blind, single-blind, studies that were in Phase 2 or Phase 3 trial were only included. Direct comparison of trials with patients treated by Bevacizumab with concurrent chemotherapy and placebo with concurrent chemotherapy in the clinical trials (phase 2 or 3) of cancer were included. The inclusion criteria of the study included the participants greater than or equal to 18 years of age, the studies which included bevacizumab plus a concurrent therapy and placebo with a concurrent therapy, the dose of Bevacizumab should be 2.5mg/kg/week for low dose regimen or greater than or equal to 5mg/kg/week for high dose regimen. The Exclusion criteria of the study included trials including patients treated previously with Bevacizumab or another similar or other malignancies within five years (unless low risk of recurrence), Also the studies with history of abdominal fistula, Gastrointestinal Perforation, intra-abdominal abscess, clinical signs or symptoms of gastrointestinal obstruction, and requirement of parenteral nutrition, non-healing wound, ulcer. Bone fracture, bleeding diathesis, coagulopathy, known CNS disease (except for treated brain metastasis), clinically significant cardiovascular disease, a major surgical procedure within 28 days of enrollment, or anticipated to occur while participating in the study were excluded from the analysis, unpublished research work or trials were excluded. The outcomes were measured for Thromboembolic events, according to National Cancer Institute Common Terminology Criteria Version 3.The outcome was measured after six cycles for six studies and till overall survival in eight studies. Data were extracted from studies meeting the above criteria. Those studies in which data was unclear asked from respective authors. In some studies, data could not obtain by the inquiry were excluded. Authors assured that the study included was only those in which allocation of both the groups were adequately randomized, and there was not any conflict of interest as well as match to inclusion and exclusion criteria. Also, the concurrent treatment was the same for the group with Bevacizumab therapy and Placebo therapy.
5. c) Statistical analysis
The outcome of the occurrence of Arterial Thromboembolic Event (ATE) and Venous Thromboembolic Event(VTE) was recorded from both the groups (Bevacizumab and Placebo), and Relative Risk (RR) was calculated with 95% Confidence Interval and funnel as well as forest plot was obtained. R version 3.3.1 (The R Foundation for Statistical Computing) was used for analysis. A P-value less than 0.05 were considered significant. The presence of small-study effects or publication bias was assessed by funnel plot and eggers value was also calculated. P-value of eggers test, >0.05 is considered to have less publication bias.
6. III.
7. Results
Total
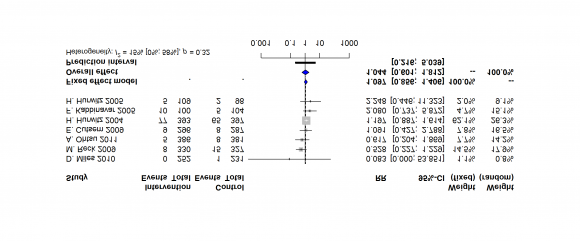
8. a) Relative Risk of Arterial Thromboembolic Events (ATE) with Bevacizumab at a low dose (2.5mg/kg/cycle) versus Placebo
There are seven clinical trials for determining the Risk of Arterial Thromboembolic Events (ATE), including 3691 patients (1866 in the Bevacizumab group and 1825 in the placebo group). The Relative Risk of Arterial Thromboembolic Events (ATE) with patients treated with Bevacizumab and concurrent therapy was 1.0974 times more than placebo and concurrent therapy with 0.856 to 1.4062 C.I and p-value is 0.4625 which is statistically insignificant. P-value of Egger's test is 0.5725.
9. b) Relative Risk of Arterial Thromboembolic Events (ATE) with Bevacizumab at a high (5mg/kg/cycle) versus Placebo
There are seven clinical trials for determining the Risk of Arterial Thromboembolic Events (ATE), including 8457 patients (4575 in the Bevacizumab group and 3882 in the placebo group). The Relative Risk of Arterial Thromboembolic Events (ATE) with patients treated with Bevacizumab and concurrent therapy were 1.6002 times more than placebo and concurrent therapy with 1.1604 to 2.2066 C.I and p-value is 0.0041which is statistically significant. P-value of Egger's test is 0.67535.
10. c) Relative Risk of Venous Thromboembolic Events (VTE) with Bevacizumab at a low dose (2.5mg/kg/cycle) versus Placebo
There are seven clinical trials for determining the Risk of Venous Thromboembolic Events (VTE), including 3691patients (1866 in the Bevacizumab group and 1825 in the placebo group). The Relative Risk of Venous Thromboembolic Events (VTE) with patients treated with Bevacizumab and concurrent therapy was 0.9143 times more than placebo and concurrent therapy with 0.7617 to 1.0975 C.I and p-value is 0.3361 which is statistically insignificant. P-value of Egger's test is 0.457.
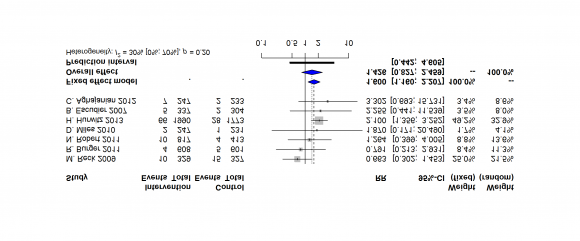
11. d) Relative Risk of Venous Thromboembolic Events (VTE) with Bevacizumab at a high dose (5mg/kg/cycle and above) versus Placebo
There are ten clinical trials for determining the Risk of Venous Thromboembolic Events (VTE), including 9379patients (5045 in the Bevacizumab group and 4334 in the placebo group). The Relative Risk of Venous Thromboembolic Events (VTE) with patients treated with Bevacizumab and concurrent therapy was 1.2243 times more than placebo and concurrent therapy with 1.0375 to 1.4448 C.I and p-value is 0.0166 which is statistically significant. P-value of Egger's test is 0.5878.
12. Assessment of Publication Bias
As indicated by the p-value of Egger's Test and funnel plots, no publication bias was reported in the selection of studies (Supplementary File).
IV.
13. Discussion
Thromboembolic events are one of the major causes of death in patients with cancer. This paper has tried to show the thromboembolic events associated with bevacizumab-: Anti VEGF at both high and low doses. The safety of this drug is still not clear due to lack of powered clinical trials. So to overcome this we have performed meta-analysis, which includes 14 randomized clinical trials including, 12,280 patients. However, many previous systematic reviews and metaanalysis showed the adverse effect of bevacizumab but not as per the dosage. In this paper, we attempted to associate thromboembolic events with bevacizumab, at different doses by using meta-analysis.
Due to the anti-VEGF effect of bevacizumab it may result in the development of venous thromboembolism.
Bevacizumab may expose subendothelial procoagulant phospholipids resulting in thrombosis by inhibiting VEGF induced endothelial regeneration and may reduce the production of nitric oxide and prostacyclin and also causes inhibition of VEGF that causes overproduction of erythropoietin that leads to increased hematocrit and blood viscosity . (21)(22)(23) Also bevacizumab could increase the discharge of procoagulant from the neoplasm into the blood due to its cytotoxic effect and also increase the expression of pro-inflammatory cytokines leading to damage and in situ thrombus formation. (24) The hallmark behind any arterial thromboembolism is that the instability of atherosclerotic plaque, activation of platelets, and decreased anti-inflammatory effect of VEGF exposure leading to plaque instability and ruptures, which leads to thromboembolism. (24,25) Our meta-analysis shows that high dose bevacizumab is related to a significant increased risk of arterial occlusion in patients who received treatment for metastatic cancers of lung, ovarian, colorectal, and pancreatic and kidney that was similar to the study of Scappaticci, Frank A., Jamey R. Skillings, Scott N. Holden, Hans-Peter Gerber, Kathy Miller, Fairooz Kabbinavar, Emily Bergsland. Our metaanalysis additionally shows the increased risk of venous occlusion with a high dose of bevacizumab that was similar to the study of Shobha rani Nalluri, David Chu, Roger Keresztes, Xiaolei Zhu, Shenhong Wu. Due to the increasing use of angiogenesis inhibitors in patients with many metastatic cancers owing to the associated survival benefit, it's important that oncologists monitor and manage these side effects befittingly to confirm that patients receive maximum benefit from bevacizumab therapy.
V.
14. Conclusion
The association of Thromboembolic events with new agents presents a challenge for recognition as a result of several RCTs might not be powered to reveal a significant relationship. Our meta-analysis has overcome B this limitation of individual trials and incontestable that bevacizumab is also related to a considerably increased risk of arterial and venous Thromboembolic events at the high dose. This finding can facilitate physicians and patients to acknowledge the danger of venous thromboembolism with the administration of bevacizumab at high doses, and so thromboembolic events ought to be monitored.






| Year 2020 |
| 72 |
| Study Name | Trial Phase | Underlying Malignancy | Bevacizumab Dose | Concurrent Treatment | ||
| A .Ohtsu 2011 et al 6 | 3 | Advanced gastric cancer | 2.5mg/kg/every week | Fluropyrimidine-Cisplatib | ||
| B .Escudier 2007 et al 7 | 3 | metastatic renal cell carcinoma | 5mg/kg/week | interferon Alfa | ||
| C .Aghajanian 2012 et al 8 | 3 | Peritoneal, or Fallopian Tube cancer. Recurrent Epithelial Ovarian, Primary | week 5mg/kg every | gemcitabine plus carboplatin; | ||
| C .Zhou 2015 et al 18 | 3 | Recurrent Non squamous non small cell lung cancer | 5mg/kg every week | Pacitaxel or carboplatin | ||
| D .Miles 2010 et al 9 | 3 | HER 2-metastatic Breast cancer | 2.5mg/kg/week | Docetaxel | ||
| D .Miles 2010 et al 9 | 3 | HER 2-metastatic Breast cancer | 5mg/kg/week | Docetaxel | ||
| E .Cutsem 2009 et al 10 | 3 | Metastatic Pancreatic Cancer | 2.5mg/kg/week | Gemcitabine and erlotinib | ||
| F .Kabbinavar et al 2005 | 11 | 2 | Metastatic Colon Cancer | 2.5mg/kg every week | Bolus fluorouracil and leucovorin | |
| H. Hurwitz 2004 et al 12 | 2 | Metastatic Colon Cancer | 2.5mg/kg/week | Irinotecan, bolus fluorouracil and leucovorin | ||
| H .Hurwitz 2005 et al 13 | 3 | Metastatic Colorectal Cancer | 2.5mg/kg/week | irinotecan/fluorouracil/leucovorin | ||
| H .Hurwitz 2013 et al 14 | 3 | Metastatic Colorectal Cancer | 5mg/kg/week | Chemotherapy | ||
| H .Kindler 2010 et al 19 | 3 | advanced pancreatic cancer | 5 mg/kg/week | Gemcitabine | ||
| H .Kindler 2012 et al 20 | 2 | Malignant Mesothelioma | 5mg/kg every week | gemcitabine cisplatin | ||
| M .Reck 2009 et al 15 | 3 | Nonsquamous Non-Small-Cell Lung Cancer | 2.5mg/kg every week | Cisplatin and gemcitabine | ||
| M .Reck 2009 et al | 15 | 3 | Nonsquamous Non-Small-Cell Lung Cancer | 5mg/kg every week | Cisplatin and gemcitabine | |
| N .Robert 2011 et al 16 | 3 | HER 2-locally recurrent or metastatic Breast cancer | 5mg/kg every week | Capecitabine taxane anthracycline | ||
| R. Burger 2011 et al 17 | 3 | Ovarian Cancer | 5mg/kg every week | Carboplatin Pacitaxel | ||