1. Introduction
ice bran is a major cereal agricultural by-products in rice-based agricultural countries like Bangladesh and has the potential as a feed ingredient. However, its utilization, especially for poultry is limited. The limitation of its use was due to its high fiber content, low protein and antinutritional factors such as Phytic acid as phytate. These antinutritive factors have been reported by Khalique et al., (2003) cause reduction of feed intake and depressed performance of broiler.
Nutritionally, several factors limited its use in poultry, especially broiler chicken diet. Almost half of phosphorous are in phytates form. Hull adulteration is a factor reducing the quality of rice bran (Farrell, 1994). High level of ash content indicates high level of hull (Warren and Farrell, 1990). Previous researches had attempted to use different techniques like fermentation (wizna et al., 2012), enzyme supplementation (Tirajoh et al., 2010) and the inclusion of fermented product (Kompiang et al., 1995) in increasing rice bran utilization for poultry feed.
Fermentation is one of the most advantageous approaches to improve the nutritive value of rice bran (Hardini, 2010). Microorganisms induced fermentation processes transformations of their metabolic activity and also increase the availability of nutrients in raw materials (Pelizer, Pontieri, & Moraes, 2007) which has been widely adopted to develop novel functional ingredients because this process may promote their functional quality such as antioxidant (Lee et al., 2008;Hardini, 2010;Wang et al., 2011;Cao et al., 2012;Kim et al., 2012) and optimize the use of rice bran in poultry feeding. Bidura et al., (2012) found that inclusion of yeast (Saccharomyces cerevisiae) increases the bioavailability of minerals and nutrients of rice bran and increase growth performance of male bali duckling. Also, fermentation of rice bran with Aspergillus niger caused change of nutrient content as poultry feed (Hardini, 2010).
Rice bran consisting of cellulose as the major component composed of cellulose, hemicellulose and lignin are coarse fiber which has some the limitations of the use of rice bran as feed in the broiler due to lack of lignocellulosic enzymes producing by digest tract but enzymes can be aided to hydrolyze the cellulose. This is different to ruminants (cattle, sheep, goats), rumen microbes producing lignocellulosic enzymes help the degradation of cellulose and hemicellulose (Muthukrishnan, 2007) by the species of cellulolytic bacteria which are Fibrobacter succinogenes, Ruminococcus flavefaciens and R. albus (Julliand et al., 1999;Koike et al., 2000;Chen and Weimer, 2001;Koike and Kobayashi, 2001). Cellulolytic ruminococci play a major role in the breakdown of plant cell wall material in the rumen (Bryant et al., 1958;Dehority et al., 1967;Sijpesteijn et al., 1951; that effectively reduced fiber and increased crude protein from corn stacks with the supplementation of Urea (3% w/w) and Molasses (5% w/w) (Gado et al., 2007;Supyiyati., 2012) due to effect of the non-protein nitrogen contribution from urea (Fontenot et al., 1983) also serves an important role in the metabolism of nitrogen-containing compounds by animals (Wizna et al., 2012) that increases the crude protein content of feed materials including rice milling waste (Amaefule et al., (2003).
In this present study, a fermentation technique was used in an attempt to improve the quality of rice bran. Ruminococcus sp. was used as the inoculum since it had been reported to produce the various cellulosomal types of enzyme complex which possesses a potential to degrade fiber (Flint et al., 1997) supplementation with urea (3% w/w) and molasses (5% w/w) which supports fermentation media and stimulate the growth of microorganisms to change the nutritional value of rice bran.
2. II.
3. Materials and Methods
The present study was carried out at the Department of Microbiology and Hygiene, Faculty of Veterinary Science and Department of Animal Nutrition, Faculty of Animal Husbandry, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh.
4. a) Bacterial Culture
Rumen ingesta was obtained through a permanent rumen fistula from the Sahjalal Animal Nutrition Field Laboratory to the analytical laboratory of the Department of Animal Nutrition Bangladesh Agricultural University, Mymensingh-2202 in strictly anaerobic conditions within half an hour for further processing.
Rumen liquor was obtained approximately 8hr. after feeding, strained through two thicknesses of cheesecloth, and collected in a 500 ml. centrifuge bottle. Air was excluded by completely filling the bottle, and closing it with a rubber stopper. The bottle was then held overnight at 2 o C and centrifuged at 1200 g for 10 min. before use.
Samples of rumen contents were 10 fold serial diluted in pre-reduced anaerobic diluents solutions (ADS) in the serum bottle with rubber stopper by anaerobic techniques up to 10 -8 dilution (Hungate, 1966) then samples were cultured into the pre-reduced specific media contained serum bottle for rumen bacteria using 1mL syringe; Rumen Fluid Glucose Cellobiose agar (RGCA) medium which was prepared under continuous CO 2 flow and incubate in Anaerobic Jar (OXOID, England) at 39 o C for 48 hours. Commercial CO 2 was freed from 0 2 , by passing it over heated reduced copper gauze. The RGCA growth media contained: 15 DNA Isolation and PCR Amplification: Total DNA extraction was performed with the QIAamp DNA Stool Kit (QIAGEN, Germany). Species-specific primer sets that amplify 16S rRNA of Ruminococcus albus, available to detect these species in rumen microbial ecosystems (Tajima et al., 2001;Koike and Kobayashi, 2001). The PCR mixture was performed using 1X PCR buffer (60 mM Tris-SO 4 pH 8.9, 18mM ammonium sulphate), 0.25mM dNTPs, 2mM MgSO 4 , 0.2 mM primer, 1U of Platinum Taq High Fidelity (Invitrogen, USA), 20ng of genomic DNA and DNA/RNA free water adjusted to a total volume of 50?L. The PCR condition was 95°C for 5 min followed by 30 cycles of 94°C for 30 sec/cycle for denaturing, annealing at 60 o C(Table 1) for 30 sec and finally 68°C 45sec for elongation, using a PxE 0.2 thermal cycler (Thermo electron corporation, USA). The PCR products were separated by 2% agarose gel electrophoresis using the molecular weight marker 100bp Ladder (Promega, USA) and the image was captured with a gel image analyzer. The purified PCR product was stored and will be sent for sequencing. The isolates were again confirmed by using the specific primer of bacteria.
The DNA fragments of the expected size (Table 1) were amplified from all the samples tested a representative image of the amplification after gel electrophoresis is shown in Figure 1.
5. ) Fermentation of Rice Bran
Rice bran was used throughout the study and was gathered from a local market and screened to remove any impurities and dirt through a sieve. It was kept in a clean polythene bag in the laboratory until used. Rice bran was diluted using carbonated water to get different moisture content at 60% level. 10% bacterial inoculum (on DM basis) were added in the diluted rice bran mixed with 2% urea (UFRB), 5% molasses (MFRB) and 2% urea plus 5% molasses (UMFRB) separately or combinedly. Anaerobic fermentation continued for a period of 48 hours at 39 °C in sealed serum bottle. After fermentation of fermented rice bran was immediately transferred to the refrigerator to stop further fermentation. pH, Proximate components (CP, CF, ADF, NDF and Ash), Total-P and Phytate-P were determined before and after fermentation of rice bran in accordance with AOAC (2005). These are the fermentated groups; RB: Rice Bran (control), RBB: Rice Bran treated with Ruminococcus sp. UFRB: Rice Bran treated with 2% urea using Ruminococcus sp. MFRB: Rice Bran treated with 5% molasses using Ruminococcus sp. UMFRB: treated with 2% urea & 5% molasses using Ruminococcus sp.
6. c) Chemical analysis
The proximate analysis of ingredients was measured by AOAC (2005). The crude protein content was measured by macro Kjehdahl digestion unit using Kjeltec 1030 and Auto analyzer procedure using autoanalyzer. Total phosphorus was measured according to AOAC (1980) and Phytate-phosphorus was determined according to Latta and Eskin, (1980).
7. d) Statistical analysis
All variables were subjected to analysis of variance (ANOVA) (Duncan, 1955) in a completely randomized design (CRD) by the statistical package using statistical computer package program (SPSS). Tukey pairwise comparisons were used to compare treatment means (Steel and Torrie, 1980).
8. III.
9. Results and Discussion
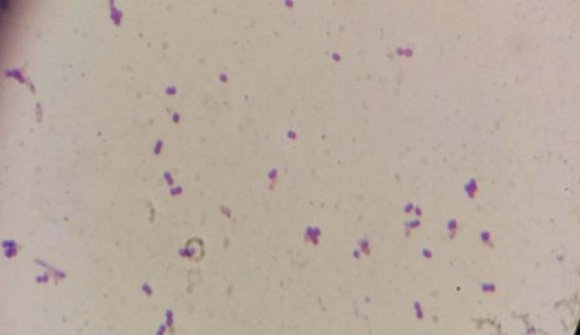
According to Morphological characteristics they were all gram positive coccoid and showed catalase & indole negative, cell arrangement were single or diplococci belong to the genus Ruminococcus sp. (Bryant et al, 1959) (figure -2). This bacterium including species (R. albus) was confirmed identified by molecular techniques (Koike and Kobayashi, 2001) and used for the fermentation of rice bran. Our Study observed that pH changes from 6.62 to 5.35 which were decreased. Results indicate that the phytate degrading enzymes from rice bran were active in the first six hours of the process. The pH changes during production of phytase in the rice bran media over 10 weeks were observed. Initial 3 weeks, a reduction in pH from pH 6 to pH 4.2 (Abd-ElAziem Farouk, 2017). The optimum initial pH for phytase production of B. cereus was pH 7.2 (Vohra and Satyanarayana, 2003). pH changes are considered to be due to the production of sugar molecule to an equimolar mixture of organic acids, ethanol and carbon dioxide by fermentation and the period of microbial growth during fermentation (Mackenzie, et al., 1965;Prabhu, et al., 2014).
In this study after 48 hours anaerobic fermentation of rice bran with Ruminococcus albus, the data of Table-2 showed that the crude protein was significantly increased in UFRB (18.43%), UMFRB (17.19%) than control RB (14.42%) but decreased in RBB (13.99), MFRB (13.20%). The highest crude protein was found in UFRB (17.19%) (p<0.05). On another hand, the data of table-02 clearly showed that crude fiber and phytate-P content was significantly decreased in all the treated groups RBB, UFRB, MFRB and UMFRB than RB control. The lowest crude fiber was found in UFRB (9.92%) (p<0.05).These results indicate that the cellulytic bacteria of rumen can improve the quality of rice bran that increased the CP with the addition of urea and molasses. The result also supported that rice bran contain cellulose as the major component, which is best for the growth of microorganisms and the production of single cell protein biomass (Yunus et al., 2015;Khin et al., 2011) which increase the crude protein content of rice bran (Sukaryana, 2001) with the addition of urea in the UFRB using cellulolytic bacteria B. amyloliquefaciens as an inoculum improved fermentation and its microbial population (Wizna et al. 2012). Protein content was also increased after fermentation of cassava waste Supriyati (2002) that agree with the result of the present experiment as protein content was increase when urea and molasses were added during fermentation (Supriyati and Kompiang, 2002). In this study, UFRB showed highest CP (18.435). Ruminococcus sp. produces the various cellulosomal type of enzyme complex which possesses a potential to degrade fiber . In this study, crude fiber was decreased in rice bran using R. albus which supports the results of Galil (2008), using bacterial treatments (Ruminococcus albus and Cl. cellulovorans) caused increases crude protein (from 1.45 to 15.16) and decreases in crude fiber (from 44.08 to 28.44%) of rice straw. Wizna et al., (2009) also found that is inoculation of B. amyloliqfacience was increased enzymes activities during fermentation of cassava waste that produces many kinds of enzymes to decrease crude fiber.
On the other hand, MFRB and RBB could not increase crude protein due to lack of additional nitrogen source to grow microbes that nitrogen was a crucial component needed by ruminal microbes after carbon and oxygen (Griffin, 1991) which need a much amino acid higher.
There was a decrease in phytate-P in a definite order in UFRB (1%), MFRB (1%), UMFRB (0.82%) than RB (1.12%) control but increase in RBB (1.21%) (p<0.05). Ravindran (1995) reported that among the common feedstuff sesame meal and rice bran have the highest level of phytate but after fermentation by Ruminococcus albus, phytate-P was decreased. Yanke et al., (1998) reported that the presence of phytase activity was investigated in 334 strains of 22 species of obligatory anaerobic bacteria that decrease phytate phosphorus in fermentation of rice bran by using rumen liquor. this results also agreed with hungate (1966) that Phytate phosphorus degrades by rumen microbes.
After fermentation of rice bran, there was no significant difference in total-P, ADF and ash content (p<0.05) but the difference in numerically. However, Total phosphorus content were within the range of 1.26-1.79% reported by Ukil (1999) and 1.62-1.81% reported by Warren and Farrel (1990c). The variations in nutrient composition might be due to the sources from which the bran was obtained. The chemical composition of rice bran varies due to the variation in the milling process and adulteration with hull (Warren and Farrel, 1990a). In this study, Total phosphorus was higher than that report.
IV.
10. Conclusions
It can be concluded that under this study fermentation of rice bran using Ruminococcus albus isolate from rumen liquid from cattle might improve nutritional value i.e. increase crude protein and decrease crude fiber, Phytate-phosphorus. However, animal experiments are required to confirm the effectiveness of fermented rice bran using Ruminococcus albus.
| The ADS media contained; 350 mL distilled H 2 O, | |
| 0.1349g K 2 HPO 4, 0.1349g KH 2 PO 4 , 0.2697g NaCl, | |
| 0.02697g MgSO 4 , 0.0357g CaCl 2 ?2H 2 O, | 0.2697g |
| (NH 4 ) 2 SO 4 , 3 drops of 0.1% resazurin. After boiling and | |
| cooling, slowly add 0.9g Na 2 CO 3. Bubble overnight (until | |
| color turns pink). Then add 5 mL of 3% (w/v) L-cysteine | |
| hydrochloride. Continue bubbling until colorless (usually | |
| requires 1 to 4 h). Dispense to serum bottle and | |
| autoclave. | |
| Bacterium | Primer name | Sequence (5´-3´) | (°C) Annealing temp. | (bp) Product size | Ref. | |
| Ra1281 f | CCCTAAAAGCAGTCTTAGTTCG | Koike | and | |||
| Ruminococcus albus | Ra1439 r | CCTCCTTGCGGTTAGAACA | 60 | 175 | Kobayashi, 2001 |
| Fermented groups | |||||
| Parameters | RB | RBB | UFRB | MFRB | UMFRB |
| pH | *6.62 a ±0.03 | 5.44 cd ±0.01 | 6.16 b ±0.04 | 5.35 d ±0.01 | 5.62 c ±0.18 |
| Crude Protein (CP) | 14.42 bc ±0.21 | 13.99 c ±0.50 | 18.43 a ±3.30 | 13.20 c ±0.29 | 17.19 ab ±0.44 |
| Crude Fiber (CF) | 12.57 a ±0.22 | 11.64 ab ±0.41 | 9.92 b ±1.38 | 11.67 ab ±0.79 | 10.83 b ±0.09 |
| Total Phosphorus | 3.29±08. | 2.99±0.30 | 3.36±0.34 | 3.28±0.55 | 2.95±0.29 |
| Phytate-P | 1.13 a ±0.03 | 1.21 a ±0.20 | 1.00 ab ±0.07 | 1.00 ab ±0.05 | 0.82 b ±0.05 |
| Ash | 12.08±0.80 | 11.96±0.30 | 11.01±2.11 | 11.43±0.28 | 10.58±0.62 |
| ADF | 24.07±4.75 | 20.60±2.37 | 18.52±0.48 | 17.75±1.37 | 20.23±2.17 |
| RB: |