1. Paracetamol May Increase Cardiac Congenital Malformations Risk in Prediabetic Pregnancy Women
Dr. Juan Ariel Jara-Guerrero
Abstract-Hepatic fat and abdominal adiposity in early pregnancy promotes impaired glucose homeostasis in midpregnancy (De Souza, 2016) and paracetamol overuse predisposes to liver fat. Hyperglycemia induces apoptosis in myocardium, and an amount evidence have demonstrated an increased risk of congenital abnormalities with gestational diabetes.
Acetaminophen overdose is the most often cause of acute liver injury and obese women are in particular risk, because is able to induce mitochondrial oxidative stress (Rousar, 2012). Acetaminophen (Paracetamol) usual high doses decreased embryonic hepatic antioxidant systems as glutathione, that play a vital role in the detoxification of exogenous and endogenous chemicals (Mitchell, 1973, Ishibashi, 1997, Beck, 2001, Rousar, 2012, rev).
The apparent safety of Paracetamol drug, a useful analgesic only (with no anti-inflammatory properties) (Neto, 2004;Hamlyn, 1978, Ucheya, 2006, Bessems, 2001) is compromized by its widespread and extensive chronic use, particularly in Peruvian population. Paracetamol though considered safe at a considerable low dose, especially in women, could cause kidney derangement and cardiac malformations during pregnant state (Ucheya, 2006), if the drug is ingested in the first trimester. Major congenital malformations, including those affecting the cardiovascular system, remain the leading cause of mortality and morbidity in infants of diabetic mothers (Pinter, 2001). Thus, there is an overcome potential maternal acetaminophen (paracetamol) toxicity (Horowitz, 1997).
2. I.
3. Hyperglycemia and Cardiac Malformations
rospective and retrospective cohort studies have demonstrated an increased risk of congenital abnormalities with gestational diabetes. This observation is related to the inclusion of women with unrecognized type 2 diabetes (Hoet, 1962, López-Quijada, 1974, Navarrete, 1970, Allen, 2007, Pasarella, 2013). It is known that glucose-induced malformations in animal models, including retarded growth and abnormal heart development, depend on the developmental stage of hyperglycemic exposure and glucose concentration (Roest, 2007, Scott-Drechsel, 2013).
Diabetes mellitus in pregnancy is associated with an increased incidence of various congenital anomalies that occur during organogenesis. Pregnancy in itself is diabetogenic as a result of an augmented physiological insulin resistance (Wu, 1996;Jara, 2001). Thus, pregestational diabetes is a major risk factor of congenital heart defects (CHD).
Animal and human confirmed a role for the diabetic state and elevated insulin resistance in inducing congenital fetal malformations, and free radicals excess have been compromised by week 8 of gestation. In this way, the major congenital malformations associated with non-physiological insulin resistance in pregnancy are caudal regression and renal, cardiac and central nervous system abnormalities (Ryan, 1998, rev).
Pregestational diabetes resulted in CHDs in 58% of the offspring, including ventricular septal defect (VSD), atrial septal defect (ASD), atrioventricular septal defects (AVSD), transposition of great arteries (TGA), double outlet right ventricle (DORV) and tetralogy of Fallot (TOF). Treatment with N-acetylcysteine (NAC) in drinking water in pre-gestational diabetic mice completely eliminated the incidence of AVSD, TGA, TOF and significantly diminished the incidence of ASD and VSD (Moazzen, 2014). It has been demonstrated that maternal hyperglycemia caused a dilation of lategestation fetal ventricular chambers, a reduction of total ventricular myocardial area, and an increase in transversal ascending thoracic aortic area (Gutierrez, 2007).
Intensive care of the pregnant mother with diabetes has dramatically decreased the incidence of diabetic embryonic malformations and clinical reports seem to link facial malformations to an increased incidence of sacral-caudal malformations in human diabetic pregnancy (Eriksson, 1985, Pinter, 1986). Indeed, congenital malformations in experimental diabetes can be prevented by antioxidants, in vivo (Simán, 1997, Reece, 1997) that replace the glutathione depletion in mother. Improving the embryonic capability to scavenge oxygen radicals, either by increasing superoxide dismutase activity or by supplying a ratelimiting precursor (N-acetylcysteine) for the enhanced synthesis of reduced glutathione, blocks the embryonic dysmorphogenesis (Wentzel, 1997).
Hyperglycemia may increase adult heart myocardial apoptosis (Gutierrez, 2009). There has been extensive evidence: maternal hyperglycemia is an inducer of birth defects that include a high incidence of cardiovascular malformations (Reece, 1997;Gutierrez, 2009).
4. II. Maternal Diabetes Mellitus may Cause Teratogenesis
Maternal diabetes mellitus is associated with increased teratogenesis risks, which can occur in pregestational type 1 and type 2 diabetes. Cardiac defects and neural tube defects are the most common malformations observed in fetuses of pre-gestational diabetic mothers (Corrigan, 2009). In addition, in nondiabetic populations, there is several reports that support the powerful association between maternal obesity and risk of congenital heart defects, this association was present not only for congenital heart defects as a group, but for numerous individual defects (Mills, 2010).
The significant correlation between maternal hyperglycemia in early pregnancy and the risk of fetal abnormalities in pregnant women with diabetes mellitus, particularly type 1 (Todorova, 2005) and non-knowntype 2 diabetes mellitus is in line with several reports that demonstrated that fetal abnormalities are strongly associated with higher levels of glycosylated Hemoglobin in the first trimester of pregnancy. (Todorova, 2005), and this elevation in A-1c Hemoglobin has a direct correlation with hepatic glutathione levels (Sakamaki, 1999) .
In this way, maternal hyperglycemia is thought to be the primary teratogen, causing particularly adverse effects on cardiovascular development. (Corrigan, 2009). Fetal cardiac defects are associated with raised maternal glycosylated hemoglobin levels up to five times more likely in infants of mothers with pre-gestational diabetes compared with those without diabetes: this cardiac defects include transposition of the great arteries, mitral and pulmonary atresia, double outlet of the right ventricle, tetralogy of Fallot, and fetal cardiomyopathy (Corrigan, 2009).
Despite improvements in prenatal care, the incidence of congenital malformations in diabetic pregnancies is still 3-4 times higher than in normal pregnancies. These defects could be attributed to alterations of intrauterine environment due to disorder of the maternal metabolism (Giavini, 1991). Glutathione (GSH), a tripeptide implicated in cellular protection against reactive oxygen species, is involved in diabetesrelated embryotoxicity Interestingly, teratogenicity of maternal serum in diabetic pregnancy is not mediated exclusively by increased concentrations of glucose and ketone bodies as its demonstrated by Wentzel et.al (Wentzel, 1996, Wentzel, 2008). Both genetic background and obesity influence the severity of fetal abnormalities in animals (Corrigan, 2009).
Previous studies in vivo and in vitro have suggested that the oxidative metabolism of the embryo may have a role in the teratogenicity of diabetic pregnancy. In particular, the production of reactive oxygen species by the embryonic mitochondria has been implicated in the teratological process. The induction of congenital malformations by the diabetic milieu occurs during the early embryonic development (Yang, 1995). Hyperglycemia during organogenesis has a primary deleterious effect on yolk sac function with resultant embryopathy (Pinter, 1986).
Diabetes-induced malformations have been often related, both in vivo and in vitro studies, to morphological and physiological alterations of the yolk sac, the principal organ for the passage of nutrients from the mother to the rodent embryo (Giabini, 1993). The embryos explanted from diabetic mothers showed signs of developmental retardation and 16% were morphologically abnormal (Menegola, 1996). In addition, reduction in embryonic GSH could reduce the protection against the oxidative stress condition described in diabetic pathology.
Norbert Freinkel suggested that the altered fuel mixture offered to the growing conceptus may be the key to most of the changes in the embryogenesis of diabetic pregnancy. He coined the term fuel-mediated teratogénesis. In vitro (Eriksson, 1991) and in vivo, a high glucose concentration causes embryonic dysmorphogenesis by generation of free oxygen radicals. An enhanced production of such radicals in embryonic tissues is directly related to an increased risk of congenital malformations in occult diabetic pregnancy.
In this concept, an abnormal handling of reactive oxygen species (ROS) is involved in diabetesinduced dysmorphogenesis in vivo. Indeed, an increased concentration of lipid peroxides, indicating damage caused by ROS, was found in fetuses of diabetes rats. In addition, embryos of diabetic rats had low concentrations of the antioxidant vitamin E compared to control embryos (Simán, 1997 a, b). On the whole, in vivo and in vitro experiments indicate that hyperglycemia itself is not a major factor in producing diabetic embryopathies (Giavini, 1993), but several depletion of tissues glutathione does it. In this way, antiteratogenic effects of supplementation of Nacetylcysteine in vitro has been demonstrated: Nacetylcysteine limits the teratogenicity of glucose (Wentzel, 1997, Roes, 2007).
There is a solid experimental, clinical and epidemiological evidence that support that increased glucose levels caused embryonic mal development in both normal and diabetic serum. Moreover, despite normalization of the diabetic state, the serum from the insulin-treated diabetic rats caused more growth retardation than the nondiabetic control serum (Wentze, 1996).
Therefore, the pathogenesis of fetal malformations in diabetic pregnancy is multifactorial. Thus, maintaining metabolites from all nutrient classes at a normal level may be important in preventing adverse fetal outcome (Eriksoon, 1993;Styrud, 1995) In the second part of gestation, the rate of heart myocardial apoptosis may increase in adult mice under a hyperglycemic environment (Frustaci, 2000, Fiordaliso, 2001, Cai, 2002)
5. Paracetamol has a Potential Teratogenic Agent
Glutathione, severely depleted in in vitro and in vivo models of diabetes (Trocino, 1995, Moazzen, 2014) is probably, the main causality factor in cardiomyocyte apoptosis (Ghosh, 2005), in addition the hyperglycemia, and both of them potentially occur, even, at usual highdoses of paracetamol.
Teratogenic potential of diabetes may consist of two components; one associated with 'direct' teratogens perturbing developmental processes in embryos at a 'critical moment' in organogenesis, and a second component, associated with a direct or indirect influence of the diabetic environment on developmental processes in the preimplantation embryos. Thus, there is a threshold glucose level associated with a clear increase of the number of litters with severely malformed fetuses in diabetics animals (Torchinsky, 1997): maternal hyperglycemia altered morphology of the lategestation fetal mouse heart (Gutierrez, 2007).
Mitochondrial alterations produced by oxidative stress have been described in embryos developing in a diabetic environment. Interestingly, its been demonstrated recently that Paracetamol in normal doses may be able to induce mitochondrial oxidative stress (Rousar, 2012). In this regard, oxygen radicalsscavenging enzymes potentially reduce the embryotoxic effects induced by diabetic conditions (Menegola, 1996).
Acetaminophen overdose is the most often cause of acute liver injury. The toxic mechanism is linked to formation of an active metabolite that reacts with glutathione generating acetaminophen-glutathione conjugate (APAP-SG). This compound has been recognized to be non-toxic generally. Recent studies showed, however, that APAP-SG could possess a toxic effect too (Rousar, 2012), particularly in visceral / abdominal obese women.
Liver glutathione stores become depleted with paracetamol (Kaneo, 1994) overdoses -chronic use-so that the liver is unable to deactivate the toxic metabolites. In fact, the paracetamol induced renal damage in pregnant women results from a mechanism similar to that which is responsible for hepatotoxicity (Sule, 2006); this mechanism is probably participate in cardiac malformations in an early pregnant state.
In this context, the worldwide use of paracetamol as a household analgesic, including during pregnancy may be dangerous: in fact, fetal tissues (and maternal) can be adversely affected by paracetamol (Neto, 2004), and are potentially dangerous in the presence of chronic abdominal obesity (pathological insulin resistance) (Jara, 2001) where liver and tissues antioxidant glutathione (Reed, 1984) is reduced.
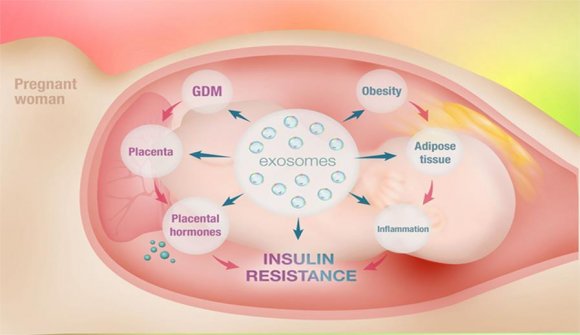
6. Prevention: The Exosomes in Pregnancy Cardiac Malformations
Animal models have been used to study the expression patterns of many genes that contribute to structural defects in the heart, although <10% of these underlie congenital cardiac defects in humans (Andersen, 2014, Richards, 2010, Govindsamy, 2018). Intrauterine under-or overnutrition alters offspring cardiac structure and function.
The expression of cardiac-specific genes is likely altered reflecting impaired cardiac insulin signalling that contributes to cardiac insulin resistance, that often precedes cardiovascular disease (Govindsamy, 2018), this hormonal event explains the fact that congenital malformations occur despite good glycemic control (Mill, 1988;Simán, 1997), thereby confirming a role for reactive oxygen species ROS and inflammatory and oxidant environment (Simán, 1997), or a lack of antioxidant protection enough, as glutathione, Vitamin E and Selenium.
Enhancing synthesis of reduced glutathione blocks the embryonic dysmorphogenesis (Wentzel, 1997), but not in cardiac malformations, because fetal heart was found to be hypertrophic (Simán, 1997) (resorption rate in fetal organs tended to be decreased with the increased dosage of vitamin E). Then, maternal administration of vitamin E can prevent congenital malformations in mid and late pregnancy, not in the early pregnancy. In comparison, Selenium may offer other additional advantages: its powerful antioxidant properties preserve reduced glutathione because its role in the major intracellular antioxidant enzyme the glutathione peroxidase (Takahashi, 1986). But Peruvian mothers don´t consume diary selenium nor vitamin D.
In addition, it is been demonstrated that acute glutathione depletion causes severe hypertension in normal rats (Vaziri, 2000): Since Paracetamol causes physiological reduction of glutathione, we don´t surprise that this drug may increase blood pressure in advanced insulin resistant humans (Sudano, 2010). Therefore, pregnant women must be caution before taking this drug, but never without medical indication.
Placental cells can communicate with maternal tissues to regulate their biological function via extracellular vesicles with immune responses: the exosomes (Salomon, 2017, rev). Acute hyperglycemia in gestation may disturb exosomes signaling , and may promotes severe malformations that occurs during organogenesis -completed by sixth week after conception-and the critical period for malformations is almost over by the time pregnancy becomes clinically apparent. At this time, the placenta releases exosomes into the maternal circulation -Exosomes are specifically package with signaling molecules, including protein, messenger RNA, microRNA, and noncoding RNA- (Mitchell, 2015, rev), and elevated maternal exosomes in gestational diabetes strikingly increased the risk of congenital heart defect (Nair, 2018;Shi, 2017), and these occurs at high amounts even in pregestational maternal diabetes that induces congenital heart defects (Kone?ná, 2019; Zhong, 2018, rev).
Exosomes released from placental cells and adipose cells have been found to be regulated by oxygen tension and glucose concentration, and abdominal obese women release an excess of this extracellular vesicles that stimulate cytokine release from endothelial cells that increase hepatic and systemic inflammation; a lot of these events disturb placental health and fetal organogenesis in the presence of glucose intolerance exacerbate in paracetamol women consumers.
In addition ro paracetamol-side-effects at normal doses, a recent investigation has found transcriptomic changes in full-genome human miRNA expression and immune modulating effects and oxidative stress responses to paracetamol even at low doses (Jetten, 2012). Because it is very frequent that organogenesis is completed before women recognize that they are pregnant, the precounseling and strict planning of pregnancy, even in prediabetic women or gestational diabetes, become an urgent need.
7. IV.
8. Conclusions
Glycolysis regulates cardiometabolism behavior during cardiac wall morphogenesis, and any disturb in metabolism pathway may alter ventricular wall morphogenesis and cause congenital cardiac malformations (Fukuda, 2019). Furthemore, if a reduction of glutathione levels in cells has been found to increase the risks for diseases and poisoning (Honda, 2017), Paracetamol, is potentially dangerous in pregnant women.
Pre-gestational maternal diabetes is associated with strong teratogenic effects on the kidney, urinary tract, and heart, and strongly associated with multiple congenital abnormalities. Pre-conception recognition of women at high risk for type 2 diabetes and optimal glycemic control may reduce the risk of congenital anomalies. A healthy diet and regular exercise (Nielsen, 2005, Allen, 2007, rev) and reduction in paracetamoltoxicity in a subclinical way, may help optimize prepregnancy weight and reduce the risk of congenital anomalies. Paracetamol may increase the risk of cryptorchidism and asthma during childhood as well as preeclampsia, maternal phlebothrombosis and pulmonary embolism (Burdan, 2012) as its recently reported.
In the presence of established diabetes, It is must be stress the importance of a strict metabolic control, started well before conception, to prevent excess rates of congenital malformation, and the intensive insulin therapy must be considered as an first option in this regard (Fuhrmann, 1984). Sustained hyperglycemia reduced endocardial and myocardial cell proliferation in the outflow tract of heart and lead to congenital heart malformations (Scott-Drechsel, 2013, Roest, 2006).
In diabetic women who is thinking to pregnant, an optimal metabolic control must been established (Todorova, 2005), and the danger of paracetamol use must be informed, even if the fact that most pregnancies are not recognized clinically until ?2 weeks after conception, thus, many pregnant women are unaware of both their diabetes status during early pregnancy and the increased risk of morbidity and mortality that prediabetes or occult diabetes has on their unborn child (Roman, 2011rev, Scott-Drechsel, 2013).
Acetaminophen (Paracetamol) is one of the most common causes of poisoning worldwide, in particular in the patients with low amount of the hepatic glutathione (Burdan, 2012, rev.), that is, insulin resistant and diabetic patients.
| Stainier DY Metabolic modulation regulates cardiac | antioxidant system in the rat embryo during early | ||||||
| wall morphogenesis in zebrafish. Elife. 2019 Dec 23; | organogenesis. Free Radic Biol Med. 1997; | ||||||
| 8. pii: e50161 | 22(3):447-54. | ||||||
| 24. Giavini E, Broccia ML, Prati M, Domenico Roversi G. | 35. Jayabalan N, Nair S, Nuzhat Z, Rice GE, Zuñiga FA, | ||||||
| Diet composition modifies embryotoxic effects | Sobrevia L, Leiva A, Sanhueza C, Gutiérrez JA, | ||||||
| induced by experimental diabetes in rats Biol | Lappas M, Freeman DJ, Salomon C Cross Talk | ||||||
| Neonate. 1991; 59(5):278-86. | between Adipose Tissue and Placenta in Obese and | ||||||
| 25. Giavini E. Diabetes in pregnancy: experimental | Gestational Diabetes Mellitus Pregnancies via | ||||||
| aspects Ann Ist Super Sanita. 1993; 29(1):27-34. | Exosomes. Front Endocrinol (Lausanne). 2017 Sep | ||||||
| 26. Ghosh S, Pulinilkunnil T, Yuen G, Kewalramani G, | 27;8:239 | ||||||
| An D, Qi D, Abrahani A, Rodrigues B Cardiomyocyte | 36. Jetten MJ, Gaj S, Ruiz-Aracama A, de Kok TM, van | ||||||
| apoptosis induced by short-term diabetes requires | Delft JH, Lommen A, van Someren EP, Jennen DG, | ||||||
| mitochondrial GSH depletion Am J Physiol Heart | Claessen SM, Peijnenburg AA, Stierum RH, | ||||||
| Circ Physiol. 2005 Aug; 289(2):H768-76 | Kleinjans JC Omics analysis of low dose | ||||||
| 27. Govindsamy A, Naidoo S, Cerf ME Cardiac | acetaminophen | intake | demonstrates | novel | |||
| Development and Transcription Factors: Insulin | response pathways in humans Toxicol Appl | ||||||
| Signalling, Insulin Resistance, and Intrauterine | Pharmacol 2012, 259:320-328 | ||||||
| Nutritional Programming of Cardiovascular Disease | 37. Kaneo Y , Ogawa K, Tanaka T, Fujihara Y, Iguchi S | ||||||
| J Nutr Metab. 2018 Feb 1;2018:8547976 28. Gutierrez JC, Hrubec TC, Prater MR, Smith BJ, Freeman LE, Holladay SD. Aortic and ventricular dilation and myocardial reduction in gestation day 17 ICR mouse fetuses of diabetic mothers Birth Defects Res A Clin Mol Teratol. 2007 Jun; 79(6):459-64. 29. Gutierrez JC, Prater MR, Smith BJ, Freeman LE, Mallela MK, Holladay SD Late-gestation ventricular myocardial reduction in fetuses of hyperglycemic CD1 mice is associated with increased apoptosis Birth Defects Res B Dev Reprod Toxicol. 2009 Oct; 86(5):409-15 30. Hamlyn AN, Douglas AP, James O. The spectrum of paracetamol (acetaminophen) overdose: clinical and epidemiological studies Postgrad Med J. 1978 Jun; 54(632):400-4. 31. HOET JP, HOET JJ, GOMMERS A, TREMOUROUX-WATTIEZ M. Prediabetes and congenital abnormalities of the heart Rev Fr Gynecol Obstet. 1962 Apr; 57:233-47. 32. Honda Y1, Kessoku T1, Sumida Y2, Kobayashi T1, induced hepatotoxicity dependent Medical Research A protective effect of glutathione-dextran macromolecular conjugates on acetaminophen-Volume XX Issue VII Version I ( D D D D ) | |||||||
| Kato T1, Ogawa Y1, Tomeno W1, Imajo K1, Fujita | |||||||
| K1, Yoneda M1, Kataoka K3, Taguri M3, Yamanaka | |||||||
| T3, Seko Y4, Tanaka S5, Saito S1, Ono M6, Oeda | |||||||
| S7, Eguchi Y7, Aoi W8, Sato K9, Itoh Y4, Nakajima A | |||||||
| Efficacy of glutathione for the treatment of | |||||||
| nonalcoholic fatty liver disease: an open-label, | |||||||
| single-arm, Gastroenterol. 2017 Aug 8; 17(1):96. doi: multicenter, pilot study. BMC 1 , Carbajo-Pescador S 2 , Mauriz JL 2 , González-Gallego J 2 , Soares FA New therapeutic 10.1186/s12876-017-0652-3. | |||||||
| approach: 33. Horowitz RS, Dart RS, Jarvie DR, Bearer CF, Gupta diphenyl diselenide reduces | |||||||
| mitochondrial dysfunction in acetaminophen-U Placental Transfer of N-Acetylcysteine Following | |||||||
| induced acute liver failure PLoS One. 2013 Dec Human Maternal Acetaminophen Toxicity J Toxicol | |||||||
| 11;8(12):e81961 Clinic Toxicol 1997; 35: 447-451 | |||||||
| 34. Ishibashi M, Akazawa S, Sakamaki H, Matsumoto K, | |||||||
| Yamasaki H, Yamaguchi Y, Goto S, Urata Y, Kondo | |||||||
| T, Nagataki S. Oxygen-induced embryopathy and | |||||||
| the | significance | of | glutathione-dependent | ||||