1. Introduction
he prevalence of diabetes is increasing globally from 463 million in 2019 to 700 million in 2045a 51% increase 1 . While several reasons are ascribed for this rising trend including aging population, urbanization, genetic predisposition, nutrition and lifestyle transition, etc., one factor that has not received adequate attention is Gestational Diabetes Mellitus (GDM) currently coined as Hyperglycemia in Pregnancy (HIP) which is defined as any degree of glucose intolerance with onset or first recognition during pregnancy 2 . GDM may play a crucial role in the increasing prevalence of diabetes and obesity 3 . In 2019 the global prevalence of Hyperglycemia in Pregnancy (HIP) in the age group 20-49 years was estimated to be 20.4 million or 15.8% of live births 1 . They had some form of hyperglycemia in pregnancy, of which 83.6% were due to GDM 1 . Hence, it has become necessary that all pregnant women should be screened for GDM, even if they have no symptoms 4 .
2. II.
3. Intra Uterine Programming
David Barker hypothesized that all adult diseases are of fetal origin. The human body's susceptibility to "lifestyle" disease(s) was "programmed intrauterine". The Intrauterine programming, that is Gestational programming is a process whereby stimuli (hyperglycemia) or stresses that occur at critical or sensitive periods of fetal development, permanently change structure, physiology, and metabolism, which predispose individuals to disease in adult life, "Fetal Origin of Adult Diseases 5 ".
4. III. Maternal Hyperglycemia & Progeny
Exposure to a diabetic environment in utero is associated with increased occurrence of impaired glucose tolerance and a defective insulin secretary response in adult offsprings, independent of genetic predisposition to type 2 diabetes 6 . The ovum is well supplied with mitochondria but the sperm contains a few and even those few do not persist in the offspring. At fertilization it is only the nucleus of the spermatozoon that enters the ovum and thus all the cytoplasm, mitochondria and mitochondrial DNA are exclusively maternally inherited 7 . Maternal inheritance is attributed to mutation in the gene(s) present on mitochondrial (mt) DNA and is transmitted invariably by an affected mother to her progeny. The unique feature of mitochondrial (mt) DNA is its maternal inheritance 7 .
There is a great variability in fetal growth in the human, based on both genetics and environmental factors. Although one cannot control one's genes, fetal growth may be affected through alteration in the maternal environment by medical nutrition therapy (MNT). MNT plays a vital role in every stage of fetal development 8 .
5. a) Diagnosis of GDM
Universal screening for GDM has to be made mandatory as intrauterine exposure of the fetus to hyperglycemia is at a higher risk of developing glucose intolerance in the future.Unfortunately, for this there is no uniformity in the guidelines for diagnosing GDM. All the diagnostic criteria require women to be in fasting, including that of International Association of Diabetes in Pregnancy Study Group guideline (IADPSG) 9 . The concern of this guideline is that, it over diagnoses GDM without clear clinical benefit 10 . Presently, this guideline's importance is declining because even at centers that accepted IADPSG recommendation, the approach varies and needs revision for standardization of the strategy for diagnosing GDM 11 .
In this context, a study established that the twohour Plasma Glucose ? 140 mg/dl (7.8 mmol/dl) with 75g oral glucose administered to a pregnant woman in the fasting or non-fasting state, without regard to the time of the last meal was able to identify woman with GDM 12,13,14 . This "Single Test procedure" which isfeasible to perform in all resource settings has been adopted by Diabetes in Pregnancy Study Group India (DIPSI) for diagnosing GDM. National Institute of Clinical Excellence(NICE) guidelines also recommend 2hr PG ?7.8 mmol/dlas one of the diagnostic criteria for GDM based on the study performed in multi ethnic population of UK 15 . The DIPSI procedure is approved by the Ministry of Health & Family Welfare Government of India 16 and recognized by, World Health Organization (WHO) 17 , International Federation of Obstetrics and Gynecologists (FIGO) 18 & International Diabetes Federation (IDF) 19 .
6. b) Appropriate trimester for Screening
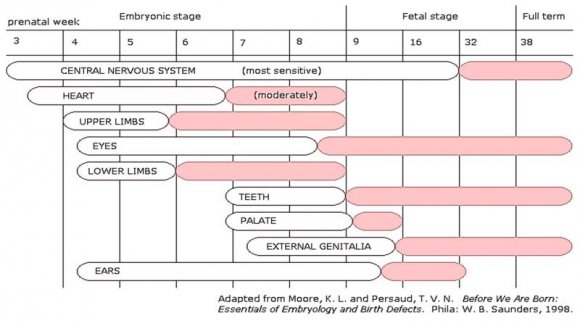
In planning for pregnancy, the metabolic state has to be maintained at normal level. During Preconception period, the recommend target glycemic level is fasting plasma glucose ? 5.0 mmol/dl, 2hr postprandial ? 6.7 mmol/dlmg/dl and A1c ? 6%. The current observation is that GDM manifests in all trimesters of pregnancy 20 .In a study, out of N=11785 screened, 31.5% were in the first trimester, 42.2% in the second trimester and 25.3% in the third trimester 21 (Table -1). The first trimester begins on the first day of the last period and lasts until the end of week 12. This means that by the time one knows for sure of her pregnancy, she might already be five or six weeks of pregnancy. By seventh week all the essential organs began to form and by ninth week almost completed (Fig- 1). A lot happens during the first three months (Fig - 2). Hence, the present recommendation is that there is a "Need for testing glucose tolerance in the early weeks of pregnancy" 22 .
In fetal pancreas each islet cell functions as an endocrine organ appears at 11 th week of gestation recognizes and responds to maternal glycemia at 15-16 weeks of gestation 23 . Fetuses are exposed to increased amniotic fluid glucose before 16 th weeks of gestation, suggesting that metabolic perturbations are underway before normally recommended 24 th to 28 th weeks of gestation for diagnosis and that earlier screening and intervention may be warranted.It is wiser to test the maternal glucose level on the next day woman misses her period. Very early diagnosis of pregnancy can be made by estimating serum beta Hydroxy Chorionic Gonadotropin (HCG) at the end of 3 rd wk of menstrual cycle and by urine beta HCG by the end of 4 th wk of menstrual cycle 24 . As alluded earlier it is advisable to screen for GDM in all the trimesters and most importantly the first trimester.
7. IV.
8. Maternal Nutrition & Target for Glycemic Control
The goal of nutrition in pregnancy is to support maternal, placental, and fetal metabolic needs, and it may be the first introduction to a lifetime of healthy eating 25 . Postprandial hyperglycemia plays a more important role in causing fetal overgrowth. Data suggests that postprandial glucose levels more closely relates to macrosomia risk compared to fasting glucose levels 26,27 . Based on studies in preterm births renal threshold for glucose in the fetus is probably <6.7 mmol/dl. When maternal glucose level is > 6.1 mmol/dl, the fetal blood glucose load causes fetal glycosuria and consequently a glucose-enriched amniotic fluid. After 20 weeks of gestation, the fetus begins to swallow the amniotic fluid. In addition to the placental transfer of glucose, ingested high glucose amniotic fluid also stimulates insulin secretion. Thus, even transient elevations of blood glucose on the maternal side not only result in elevation of blood glucose on the fetal side but also provide for glucose ingestion by the fetus for many hours. Thus, post prandial hyperglycemia for less than1 h once a day in the mother may produce fetal insulin stimulus, through the oral route for hours. Elevations of maternal glucose levels more frequently (after every meal, for example) may produce a more prolonged oral glucose load for the fetus resulting in an overfed fat fetus 28 .Monitoring maternal glycemia and maintaining 2 hr postprandial plasma glucose between 6.1 -6.7 mmol/dl by using plasma calibrated glucometer level every week, may be a wise decision.
V.
9. Conclusion
Fetal Development invariably involves exquisite interplay between maternal physiology, metabolism and hormones. Nature Nurtures the embryogenesis from conception to confinement. The environment that the oocyte is exposed to, during the peri-conception period can have a significant impact on oocyte developmental competence. The ability of the oocyte to support fertilisation and subsequent embryo development and the long-term health of the resulting offspringdepends upon the optimum metabolic control early in pregnancy. This will necessitate pre-pregnancy planning for women with pre-existing diabetes, as well as for those at increased risk of GDM, and better means to safely normalize glycemia. The aim in the management is, the "Growing fetus should not know that its mother has Glucose Intolerance". For that glycemic target should be that of normal pregnancy, the FPG 4.4 -5.0 mmol/dl, Post prandial glucose 6.1 -6.7 mmol/dl and Mean PG glucose 95 -105 mg/dl(5.3 -5.9 mmol/dl) 29 . The goal is to obtain newborn babies birth weight appropriate for gestational age between 2.5 and 3.5 kg, a step to prevent offspring developing diabetes 30 . Breastfeeding for the first 6 months protects offspring developing Type 2 DM 31 . Preventive measures against diabetes should start during intra uterine period and continue throughout life from early childhood 32 . GDM offers an important opportunity for the development, testing and implementation of clinical strategies for diabetes prevention 33 . Though the fetal development is discussed in days and weeks, it is wiser to maintain recommended target glycemic level during pre-conception period and from conception to confinement. This continued care is a "A step towards strategy for prevention of diabetes".
10. Compliance with Ethical Standards
Conflicts of Interest: All the authors are governing council members of Diabetes in Pregnancy Study group of India (DIPSI) Financial Disclosure: The authors declare that no financial assistance was received from any organization for the conduct of this study.
Funding/Support: The authors declare that no financial assistance was received from any organization for the conduct of this study.
11. Ethics Approval: Ethical Approval Obtained
Acknowledgements: Nil
| Trimester | Number of mothers screened | GDM N(%) |
| 1 | 4300 | 233 (31.5) |
| 2 | 4632 | 320 (42.2) |
| 3 | 2853 | 187 (25.3) |
| Total | 11785 | 745 (100) |