1. M
Results: A total of 93 cases were included for analysis. The Apart form a single review on the disease published by Seccia (10) on 2005, there is not another conclusive analysis of APAC cases. Many cases have been reported and published since then. We herein present a case managed in our institute, and have searched and analyzed the online data base (1955-2020) of APAC cases to review demographic characteristics, clinical and histological features, and treatment outcomes of the APAC.
2. II.
3. Case Report
A 40 years gentleman with one year history of hypertension under medication presented with left sided lumbar pain and non projectile vomiting in April 2020. He gave history of weight loss of 21 kg in past 7 months and had polydipsia. There was no history of headache, muscle weakness, loss of consciousness, chest pain or shortness of breath. He was smoker and consumes mixed diet. On examination the blood pressure was 170/100 mmHg. Other general physical and systemic examination was unremarkable. The total blood count was within normal limit. Blood sugar was 120mg/dl. The presentation and the disease stage were the significant covariates of the disease survival.
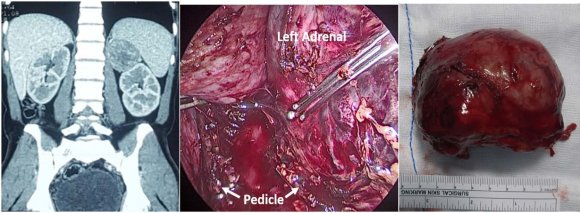
The incidence of the adrenocortical carcinoma (ACC) is 1-2 per million population per year which is more common in age group 40-50 years. Approximately half to two third of the adrenocortical carcinoma is functional and fewer than 10% present with hyperaldosteronism (5,6) Aldosterone producing adrenocortical carcinoma (APAC) is responsible for about 20% of resistant hypertension in adult and is also called as mineralocorticoid hypertension. (7) The diagnosis of APAC is based on presence of hypokalemia, high serum aldosterone level, and suppressed plasma renin activity associated with adrenal computed tomography radiographic findings suggestive of malignancy (7)(8)(9) blood sodium and potassium level was 145 meq/L and 2.0 meq/L respectively. The ultrasound abdomen revealed left adrenal mass which was confirmed by contrast computed tomography of abdomen. Contrast enhanced Computed tomography (CECT) [figure 1 (a)] revealed heterogeneously enhancing left adrenal mass that was 5x6x5.7 cm in size with well defined margin abutting the spleen, pancreas and posterior abdominal wall. The mass had displaced the left kidney inferiorly.
With diagnosis of aldosterone secreting adrenal tumor patient underwent transperitoneal laparoscopic left adrenalectomy, figure 1 (b) and (c). The intraoperative and postoperative period was uneventful. The histopathological report revealed adrenocortical carcinoma stage II with low mitotic count. The serum aldosterone level and Plasma renin activity done one week post surgery was normal. The CECT scan done 9 months post surgery had normal findings and patient was normotensive and normokalemic during the follow up.
4. III.
5. Methods
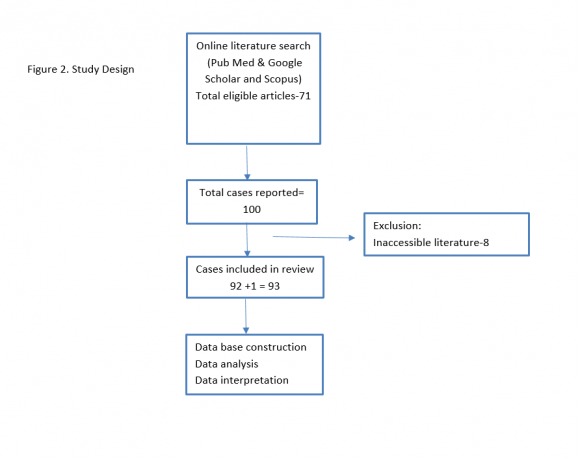
The online literature search on aldosterone producing adrenocortical carcinoma was performed through Pub Med, Google scholar, and Scopus search engine. The study design of this review is depicted in figure 2. The cited references were cross examined for APAC cases. Full article with confirmed diagnosis of adult aldosterone producing adrenocortical carcinoma were included in this review. The patient's information pertaining patient demography, clinical presentation, biochemical investigations, histopathological findings, and treatment outcomes, and follow up details were extracted. A database was then constructed for statistical analysis.
IV.
6. Results
We identified 100 cases from 71 academic articles on aldosterone producing adrenocortical carcinoma published in year between 1955 and 2020. Out of 100 only 93 were eligible for data base construction and analysis. More than half of the cases were from American region (after World Health Organization). The mean age of the study population was 45.7±15.0 (Range 17-79 years). Majority of the patients were between 17-60 years (77.4%) with male female ratio of 1:1. 4 Median disease free survival was 25 months (95% CI-16.5-33.5) and median overall survival was 36 (95% CI-18 -54). Female had higher median overall survival than male (27 vs 18 months). Patient age > 60 years had significantly lower median disease free survival (14 months) than age group ? 60 years (30 months). The overall recurrence rate was 55%; Liver (35%), Lungs (22.5%), local recurrence (15%), Bones (12.5%) and abdominal lymph nodes (12.5%). In Cox regression analysis patient age at diagnosis, and the staging characteristics (size, lymph nodes involvement, invasion and distant spread) were the significant (p=0.05) covariates of the disease free and overall survival.
V.
7. Discussion
The first case of adrenocortical carcinoma presenting with hyperaldosteronism was published in There were no enlarged lymph nodes and metastatic lesion elsewhere in the body. The serum aldosterone concentration was 535 ng/dl (normal 2.52-39.2 ng/dl) and the plasma renin activity was 0.5 ng/ml/hr with aldosterone renin ratio (ARR) 1070 ng/dl/ng/ml-hr. The serum and urinary metanephrine and nor-metanephrine was normal. The serum cortisol level was 10.6 ng/ml (normal 6.4-21 ng/ml). The summary statistics; mean ± SD, median (and range) were calculated as appropriate after testing for normality. The difference in distribution of the categorical variables were evaluated using Chi Square test and P<0.05 was considered significant. The disease free survival time was defined as the time between initial treatment with curative intent and the first radiological evidences of adrenal tumor, local recurrence or metastasis. While overall survival interval was defined as the time between initial treatment (or initial diagnosis if initial treatment date is not available or if the treatment was not offered) to censoring at death or end of study. Kaplan Meier analysis was done to plot the disease free survival and overall survival. Cox regression proportional hazard analysis was performed to evaluate the correlation between disease variables and outcomes (disease free or overall survival) 1955 by Foye (10) and Jerome W. Conn further
The demographic and biological features of the APAC remained same when we moved from series of 58 cases published in 2005 (10) to 93 cases in this review. Hypertension and hypokalemia were the most consistent sign of Conn's syndrome (17) and the later was commonly associated (83%) with muscle weakness. Contrary to the ACC in general (15), APAC was predominantly found on right side. CECT characteristics are the mainstay of diagnosing malignant adrenal mass. At cutoff of 4 cm, sensitivity and specificity of diagnosing malignant adrenal incidentaloma was 93% and 76%. (18,19) sampling for hyperaldosteronism is indicated to confirm unilateral disease if the CT scan is not abnormal, shows bilateral disease, or unilateral disease in patient age > 35 years. (25) It is not always easy to confirm benign and malignant adrenal tumors based on histopathological features. The differentiation of malignant form benign adrenal mass essentially depends on local invasion and distant metastasis. Tumor size larger than 5 cm and greater than 100 gm has malignant potential. Weiss criteria is the simple and reliable system to diagnose malignant adrenal tumor with threshold of total score ? 3. The five criteria used in the updated Weiss system include: >6 mitoses/50 high-power fields, ?25 percent clear tumor cells in cytoplasm, abnormal mitoses, necrosis, and capsular invasion. (26)(27)(28) In this review 80% (55/75) of the APAC were larger than 5 cm, 60% (24/35) were larger than 100 gm and 57 % (33/58) had mitotic count > 20/high power field. Histopathological and molecular diagnosis of APAC often has limitations. A total of three cases of adrenal tumor diagnosed as carcinoma one and half year after primary surgery. (10,29,30) There are several marker(alpha-inhibin, Melanin A, SF-1) that can identify the origin of adrenal tumor as well as differentiate the benign from malignant elaborated the spectrum of clinical features of hyperaldosteronism in 1965. (11) The largest series were published by Mouat and Kendrick in 2019 and 2002 respectively. (12,13) Aldosterone hypersecretion is the least common (0-7%) among the functional adrenocortical carcinoma (ACC). Though surgery remain potentially curative in early stage ACC, the recurrence and metastasis post surgery were common. The effect of functionality on tumor behavior and outcome is not quite predictable. (6,(14)(15)(16) The analysis and review was done to highlight and update the clinicpathological behavior of the APAC.
The characteristic CECT findings of the APAC were heterogeneous enhancement, calcification, capsular invasion, irregular margin, local infiltration, renal or inferior venacava thrombosis, and metastatic lesion. The published research showed that the unenhanced CT attenuation ?10 HU or a combination of tumor size ?4 cm and HU ?20 almost excludes adrenal malignancy. (20,21) There is increase probability of adrenal malignancy with the increase size of the tumor (55% for tumor >10 cm).( 22) However, 26.7% of the reported APAC size in this analysis were ? 5 cm and 20% (2/10) of the stage IV tumor were size ? 5 cm. Positron emission tomography (PET) scanning with fluorodeoxyglucose (FDG) is valuable tool for diagnosing malignant adrenal tumor. PET-CT (with an SUV cutoff value of 3.1) has sensitivity, specificity, positive predictive value, and negative predictive values for malignant adrenal tumor of almost 100%. (23) Radiological imaging has limitation in diagnosing tumor less than 1 cm, and bilateral tumors. A systematic review of 38 studies found inappropriate management of 37.8% of primary aldosteronism cases when diagnosis was determined by CT/MRI alone.( 24) Adrenal venous The primary hyperaldosteronism manifest with triad of hypertension, spontaneous hypokalemia, and metabolic alkalosis. Primary hyperaldosteronism is suspected when serum potassium < 3.5 meq/L, plasma renin activity less than 1 ng/ml/hr, and plasma aldosterone concentration ? 10ng/dl, and PAC/PRA >20 ng/dL per ng/mL/hour. (7,21) The combination of a PAC > 20 ng/dLand a PAC/PRA ratio > 30 ng/dL per ng/mL/hour have a sensitivity and specificity of 90 percent for the diagnosis aldosterone producing tumor (26), and was consistent findings in this study. Study says 9-37% of the primary hyperaldosteronism can present with normal serum potassium level. (27) In this review 7%-9% of APAC had normal laboratory findings (normokalemic, normal PAC and PRA level). In normokalemic but hypertensive cases the diagnosis of APAC is confirmed with additional testing (23); 24 hour urine aldosterone, sodium, and creatinine on high sodium diet, fludrocortisone suppression testing, and or saline suppression testing can confirm the diagnosis in suspicious cases.
Most of the adrenocortical carcinoma are sporadic and some are components of several hereditary cancer syndrome. (28) Less than 10% ACC present with virilization alone or feminization or hyperaldosteronism. (5,28) adrenal tumor (Ki67 proliferation index, overexpression of TP53, IGF-2, and cyclin E) however, they are not sufficiently discriminatory. (31,32). Surgery is the mainstay treatment for potentially resectable stage I to stage II adrenocortical carcinoma. (35,36) Routine lymphadenectomy had shown improved recurrence free survival and decreased disease specific death (hazard ratio [HR] 0.54, 95% CI 0.29-0.99). (37) Open surgery is recommended method of surgery for ACC. (38,39) European clinical practice guideline recommends laparoscopic surgery for adrenal tumor less 6 cm in absence of local invasion. (36) However studies had shown comparable outcome from open and adrenalectomy even for tumors upto 10 cm. (40,41) We found patient age at diagnosis and staging characteristics significant (p<0.05) predictors of disease free survival and overall survival. Metastasis at presentation; capsular, vascular, and adjacent organ invasion; tumor necrosis; mitotic activity were proven significant covariates of disease specific survival in adrenocortical carcinoma. Markers of proliferation like mitotic rate, and Ki67 expression has prognostic value as well. (42,43) Recurrence rate of APAC was 55% which is comparable with the recurrence rate of ACC (60-80%) in general. Liver and lungs were the two most common site of APAC recurrence. Adjuvant mitotane therapy has shown improved recurrence free survival and indicated for histologically high-grade disease, intraoperative tumor spillage or fracture, and some large tumors with vascular or capsular invasion. (44)(45)(46) However National Comprehensive Cancer Network (NCCN) guidelines suggest that mitotane be "considered" (category 3 recommendation) for all patients with resected low-or high-grade localized ACC regardless of stage or tumor size. (47) We found no uniform criteria of using adjuvant therapy in this study 14/93. Cytotoxic chemotherapy (etoposide, doxorubicin, and cisplatin) in combination with mitotane is suggested in rapidly progressive, high grade and metastatic disease. (48)(49)(50) Post operative radiation therapy is beneficial in local control of disease and suggested in incompletely resected ACC, stage III disease, those who have tumor spillage, and for all patient with high grade ACC. (36) VI.
8. Conclusion
APAC is a rare adrenal malignancy that requires meticulous evaluation before offering definitive surgery. Recurrence rate is high with dismal prognosis. Further research on tumor biology, natural history, and treatment outcome can add more to the understanding of this rare variant of adrenal malignancy. Declarations Funding Not applicable According to German ACC registry the fiver year disease specific survival of adrenocortical carcinoma for stages I, II, III, and IV were 82%, 61%, 50%, and 13% respectively. (33) We found that the aldosterone producing adrenocortical carcinoma had more dismal prognosis with mean and median disease free survival of 49 months and 25 months respectively. Incoherence in the stage specific median disease free survival (Stage I < Stage II) might be due to variable follow up duration of the heterogeneous and small number of cases in each stage (figure 3 and 4). Occult micro metastasis is presumably responsible for the early recurrence and disease progression. (34) In this series the recurrence rate among the size group of 0-5 cm; 6-10 cm; 11-15 cm; and > 15 cm were found to be 50%, 55%, 42%, and 100% respectively.
| Aldosterone Producing Adrenocortical | |
| Carcinoma: A Case Report and Systematic | |
| Review of the Rare Disease | |
| Bikash Bikram Thapa ? & Bina Basnet ? | |
| Abstract-Introduction: Functional adrenocortical carcinoma is very uncommon. Aldosterone producing adrenocortical | Introduction |
| carcinoma (Conclusion: Aldosterone producing adrenocortical carcinoma | ost of the adrenal tumor are benign non-functional incidentaloma.(1) Cortisol hypersecretion (9.2%) followed by pheochromocytoma (4.2%) and aldosteronoma (1.6%) is the most common hormonal abnormality in functional adrenal incidentaloma. The prevalence of the primary and secondary adrenal malignancy was 1.9% and 0.7% among adrenal incidentaloma.(2-4) |
| is one of the rare types of functional adrenal malignancy. Any | |
| suspected case should undergo thorough clinical, radiological | |
| and biochemical evaluation. Surgery is the mainstay treatment | |
| and adjuvant therapy has no conclusive role in disease free | |
| survival. A large cases series and multicenter study could | |
| further add scientific evidence for management of the APAC. | |
| hypertensive medications even after surgery. According |
| to American Joint Committee on Cancer (AJCC) 8 th |
| edition the adrenocortical carcinomas of the study |
| group were classified into Stage I-21.5%, Stage II- |
| 38.7%, Stage III-16.1%, and Stage IV-17.2%. The |
| staging details were not available in about 6.5% (n=6) |
| cases. 89% of the patient underwent surgery irrespective |
| of the disease stage. |
| 70% of the reported APAC in | |||
| this study were pure aldosterenoma and 30% were | |||
| mixed (hyperaldosteronism with hypercortisolism-20%, | |||
| hyperaldosteronism | with | hypercortisolism | and |
| hyperandogeniemia-7%, and hyperaldosteronism with | |||
| hyperandrogenemia-3%). | |||
| adenoma presented with metastatic adrenocortical | |||