1. Introduction
ertigo or dizziness is a global problem and it affects approximately 15% to over 20% of adults annually according to large population-based studies (1). Acute dizziness/vertigo is a symptom of vestibular dysfunction in brain. Thus, this syndrome is also known as acute vestibular syndrome (AVS), which is characterized by acute dizziness/vertigo, head-motion intolerance, gait unsteadiness, nausea/vomiting, nystagmus, and duration of 24 hours or more (2,3), even presenting with a transient AVS (lasting seconds to hours, occasionally days) (3,4). At any point along the vestibular pathway from the peripheral labyrinth to the central vestibular cortex, AVS may occur; the causes of AVS is divided into peripheral and central AVS (3).
Previous cases studies have described intracerebral hemorrhage (ICH) with acute vertigo or AVS (5,6). However, the frequent/ docking sites of acute ICH causing an AVS remain unknown. Head computerized tomography (CT) is commonly used as a diagnostic test for stroke with acute vertigo presentations (7). The importance of using head CT is facilitate an understanding of the precise anatomical location in the brain. The central AVS is mainly located in brainstem, cerebelum, thalamus, and cortex, but the vestibular pathway from thalamus to cortex is still well unknown. Therefore, our aim was to assess the frequent/docking sites of central AVS in acute ICH from a prospective head CT scan population.
2. II.
3. Methods and Materials a) Study settings
The study design was a prospective registered for all patients from the intensive care unit (ICU) and neurologic wards (stroke center) in the Affiliated Shuyang Hospital of Xuzhou Medical University in Northern China (January 1, 2014 through December 31, 2016). The frequent sites of central AVS were retrospectively assessed based on head CT (minority with MRI). The image docking test between thalamic vestibular station and homologous cortical vestibular organ was measured and studied. We selected the images of AVS patients with small thalamic hemorrhage as the carrier of the thalamus vestibule station, and selected the images of AVS patients with small insular or parietal/temporal hemorrhage as docking body (its cutting the horizontal line perpendicular to the edge of the hematoma, hematoma side image retention, docking with the ipsilateral craniofacial coincide). The study was approved by the local ethical committee on clinical research of the hospital, and written informed consent was obtained from the patients' families.
4. b) Patients and selection criteria
Based on the International Statistical Classification of Diseases 10th Revision (ICD-10) by the WHO (1994), we identified acute vertigo syndrome (H81.9) and central vertigo (H81.4), and we also identified patients who had acute spontaneous ICH (code I61). We retrospectively analyzed adult patients who were verified as having an acute ICH within a 3-year period from their emergency head CT scan on admission (exclusion: traumatic ICH or subarachnoid hemorrhage).
For the purposes of this study, the inclusion criteria of the central AVS due to acute ICH were as follows: ? initial rapid onset symptoms adapting AVS criteria (2-4); and ? acute ICH located in a cerebral vestibular structure or pathway confirmed by head CT on admission. We excluded patients with acute ICH without data in their medical records due to either death or moribundity within the first 24 hours. We also excluded acute ICH resulting from an underlying neoplasm or a hemorrhagic infarction.
We analyzed the CT data that were collected at the closest time following the onset of AVS. The ICH volume was calculated from the first CT scan using the a×b×c×0.5 method, as previously described (8).
5. c) Related definitions and Clinical assessment
A small ICH was diagnosed according to the following criteria (9): ? hemorrhagic volume <3 ml in the brainstem; ? hemorrhagic volume <5 ml in the cerebellar; ? hematoma volume <10 ml in the thalamus or basal ganglia; and ? hematoma volume <15 ml in the lobar.
Central AVS refers to a cause from impaired central vestibular pathways and/or lesion evidence from images of central vestibular pathways.
The hospital charts of all ICH patients with and without central AVS were reviewed by a senior author. This author compiled the clinical information about central AVS and other findings of neurological examination, including focal neurological symptoms/ signs, the bedside oculomotor examination (i.e., the head Impulse test, nystagmus assessment, and skew deviation), while also including the relationships of clinical outcomes with age, sex, days in the ICU, underlying disease, hematoma location, hematoma volume, accompanying intraventricular extension, NIHSS score, GCS score, and the onset-to-admission time.
6. d) Statistical analysis
The results from the data are expressed as mean ± standard deviation (SD) or median (IQR), and number (percentage) for qualitative values. The statistical analysis was conducted using SPSS version 17?0 (SPSS Inc., Chicago, IL, USA).
7. III.
8. Results
A total of 1393 adult acute ICH patients who come from general intensive care unit (ICU, 427/628) and neurologic ward (702/765) were prospectively recruited. After application of the eligibility/ exclusion criteria, 1129 ICH patients were included in the present investigation (Figure 1). 70 patients (6.2%) with acute ICH with central AVS were confirmed by head CT. The mean age was 64.2±13.0 years old, and median age was 63.5 years (range, 41 to 92). Among them, there were 47 (67.1%) males and 23 (32.9%) females. The median time from the onset to the hospital admission was 6.5 hours. Clinical features of 70 patients with central AVS caused by acute ICH are shown in the Table 1.
9. AVS=acute vertigo syndrome; ICH=intracerebral hemorrhage; NIHSS= National institute of health stroke scale? GCS= Glasgow Coma Scale a) Imaging change of central AVS due to acute ICH
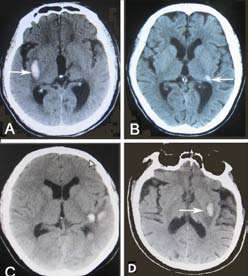
The insular lobe (15/70) (Figure 1) was one of the frequent sites of hemorrhage causing central AVS in all cerebral lobes. Of them, the left insular lobe was in 8 (53.3%) cases and the right insular lobe was in 7 (46.7%) cases. Moreover, the hemorrhage involved the posterior insular lobe cortex in 10 patients, medianinsular lobe in 2 cases, anterior-insular lobe in 2 cases, and anterior-median insular lobe in 1 case. The smallest hematoma volume was 1.3 ml, and the largest hematoma was 24.5 ml, and the median hematoma volume was 3.0 ml. The other cerebral lobe hemorrhages included frontal lobe in 3 cases, temporal lobe in 2 cases, parietal lobe in 6 cases, and occipital lobe in 4 cases.
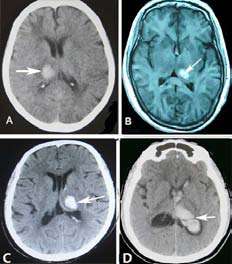
Among 15 thalamic hemorrhage cases with central AVS, the patients' head CT showed that 12 (80.0%) cases impaired the thalamic vestibular structure, which was usually limited to the posterolateral thalamus. (Figure 2) Another 3 patients had a vestibular lesion located in the dorsal thalamus, posteromedial thalamus, and global thalamus, respectively. All 15 patients contained unilateral lesions. Of them, intraventricular extension was present in 9 patients, coma or stupor in 5 cases, downward deviation of eyes in 5 cases, hemiparesis in 4 cases, numbness of limbs in 1 case. IV.
10. Discussion
Occurrence of central AVS due to infratentorial cerebellar or brainstem hemorrhage is well-known, but central AVS resulting from supratentorial vestibular hemorrhage is not well recognized.
In the present study, 6.2% of patients with acute ICH were associated with central AVS events. We found that the frequent sites of central AVS due to acute ICH were limited between the posterolateral thalamus and insular lobe cortex. Moreover, most patients had a small hematoma, suggesting that this area has a distinct vestibular docking pathway. This speculation is wellsupported by evidence from a previous study (10).
Although a previous study demonstrated that the ICH between the putamen and insular cortex accounted for 21% of hemorrhagic lesions in the striatocapsular area (11), the pure insular lobe hemorrhage causing an AVS was rarely reported. Our current data showed that the insular lobe hemorrhage was one of the frequent locations causing a central AVS.
Of them, the central AVS with a very small hematoma mainly located in the median-posterior of the long insular lobe. The posterior insular lobe hemorrhage was the most frequent site leading to a central AVS. A previous study confirmed that the primary central vestibular cortex is located in the insular cortex (12), and this has been supported by our current study of head CT.
There have only been sporadic reports of patients with cerebral lobe hemorrhage causing central AVS (13), although the cortical representation of the vestibular projections in human beings has been demonstrated in distinct temporal and parietal areas (14)(15)(16)) and the frontal lobe area (16,17) of both hemispheres. The present study showed that insular lobe hemorrhage was the most frequent site resulting in a central AVS, but the frontal, temporal, parietal lobe, and occipital hemorrhage may also present with AVS.
Thalamic hemorrhage occurred in up to 33% of patients with ICH (18). Although a previous PET study confirmed that the posterolateral thalamus is a unique relay station for vestibular input to the cortex (10). Clinically, only 2 patients with thalamic hemorrhage with AVS were reported, including 1 posterior thalamic hemorrhage (19), and 1 posterolateral thalamic hemorrhage (20). However, our current prospective imaging-based study has showed that thalamic hemorrhage is a frequent event resulting in AVS. The location of thalamic hemorrhage causing AVS was typically localized to the posterolateral thalamus. This is the first clinical confirmation via a series of thalamic hemorrhage cases that the posterolateral thalamus is a terminal area of the brainstem vestibular pathway.
Importantly, in our current study series, the images docking test confirmed that 3 cases with left posterolateral thalamic hemorrhage with AVS and 3 cases with left posterior insular hemorrhage with AVS were successfully docked in zero distance. Whereas, because of the long distance from thalamus to parietal/temporal lobe vestibular cortex, the images docking test were failed between 3 patients with left posterolateral thalamic hemorrhage with AVS and 3 patients with left parietal/temporal lobe hemorrhage with AVS. It is show that the precise docking site of vestibular pathway from thalamus to cortex is located between posterolateral thalamus and posterior insular. Therefore, one should keep the fact in mind that the posterolateral thalamus and posterior insular cortex is the frequent/docking sites resulting in central AVS.
However, the limitations of the current study were difficult to avoid. First, patients with small ICH are more likely to mimic a transient ischemic attack or have rapidly resolving symptoms (21), and some patients who suffered from a very small ICH associated transient central AVS were not sent for hospitalization. Therefore, the rate of ICH with AVS may still be underestimated. Second, the confirmed vestibular pathway between the thalamus and insular cortex was based on images docking test, but our imaging studies was still limited. Therefore, further research is necessary. In addition, the rate of nystagmus was low in this series; this is because the majority of patients with insular lobe small hemorrhage were less likely to affect oculomotor function.
V.
11. Conclusions
The frequent/docking site of central AVS is localized between the posterolateral thalamus and posterior insular cortex, suggesting that this location has a distinct vestibular docking pathway.
| Prior ischemic stroke | 11(15.7) | |
| Prior ICH | 4(5.7) | |
| Prior cardiac disease | 2(2.9) | |
| Clinical characteristics: | ||
| Insular lobe hemorrhage n(%) | 15(21.4) | |
| Thalamus hemorrhage n(%) | 15(21.4) | |
| Brain stem hemorrhage n(%) | 10(14.3) | |
| Cerebellar hemorrhage n(%) | 12(17.1) | |
| Premary intraventricular | 3(4.3) | |
| Other cerebral lobe hemorrhage n(%) | 15(21.4) | |
| Intraventricular extensio n(%) | 15(21.4) | |
| Year 2021 | SBP, (mmHg, mean ±SD) DBP, (mmHg, mean ±SD) Transient AVS n. (%) Persistent AVS n. (%) | 174.8±34.3 100.9±17.2 12(17.1) 58(82.9) |
| Initial head-motion intolerance n. (%) | 61(87.1) | |
| Volume XXI Issue II Version I | Gait unsteadiness n(%) Vomiting or nausea n(%) Spontaneous nystagmus, n(%) Nystagmus after head impulse test, n(%) Nystagmus after ocular tilt test, n(%) Non vestibular sympoms n. (%) Hemiparesis Numbness of limbs Slurred speech Downward deviation of eyes, Loss of consciousnes Drowsiness | 52(74.3) 57(81.4) 10(14.3) 12(17.1) 9(12.9) 14(20.0) 3(4.3) 5(7.1) 5(7.1) 12(17.1) 4(5.7) |
| ( D D D D ) A | Headache Hemianopsias | 6(8.6) 1(1.4) |
| Medical Research | Positive Babinski signs Admission median NIHSS score(range) Admission median GCS score(range) Mortality at 30 days, n(%) AVS=acute vertigo syndrome; ICH=intracerebral hemorrhage; SBP=systolic blood pressure, DBP= diastolic blood pressure, 13(18.6) 2(0-23) 14(8-15) 8(11.4) NIHSS= National institute of health stroke scale; GCS= Glasgow Coma Scale | |
| Global Journal of | Characteristics Median age ,yr(range) Male, n. (%) Hypertension (78.6%, 55/70) was the most common risk factor of these patients, followed by cerebral amyloid angiopathy (12.9%, 9/70). Most patients with central AVS were caused by small ICH, the most frequent symptoms of patients were persistent AVS (82.9%, 58/70), while transient AVS only occurred in 17.1% of patients. | Velue 47(67.1) 63.5(41-92) |
| Median time from onset to CT,h (range) Sixty-one (87.1%) patients with rapid onset central AVS had head-motion intolerance. 57 (81.4%) Risk factors n. (%) Hypertension Cerebral amynoid angiopathy patients had vomiting or nausea, 52 (74.3%) patients had unsteadiness, and only 31 (44.3%) patients had nystagmus. | 6.5(0.5-335) 55(78.6) 9(12.9) | |
| Diabetes mellitus | 8(11.4) | |
| Moyamoya disease | 2(2.9) | |
| Unknown | 2(2.9) | |
| Site of ICH | Cases N,(%) | Hematoma Volume, M(IQR) | GCS (mean±SD) | NIHSS (mean±SD) | Death |
| Thalamus | 15(21.4) | 4(2.5-7.5) | 11.5±4.2 | 7.7±8.2 | 4 |
| Insular lobe | 15(21.4) | 3(1.3-24.5) | 14.7±0.8 | 1.8±3.3 | 0 |
| Frontal lobe | 3(4.3) | 5.8(1.5-22.7) | 14±1.4 | 3±2.8 | 0 |
| Temporal lobe | 2(2.9) | 11.5(8-14.7) | 11.0±5.7 | 12.0±15.6 | 0 |
| Parietal lobe | 6(8.6) | 6(2-15.0) | 15±0.0 | 2±1.7 | 0 |
| Occipital lobe | 4(5.7) | 10(3.8-14.5) | 12.5±5.0 | 6.3±9.2 | 1 |
| Premaryintraventricular | 3(4.3) | N/A | 9±5.1 | 14.7±12.7 | 0 |
| Brainstem | 10(14.2) | 2(1.5-3) | 9±5.7 | 13.4±11.4 | 3 |
| Cerebellum | 12(17.1) | 3.5(3-4.9) | 13.6±1.3 | 1.2±0.4 | 0 |