1. Introduction
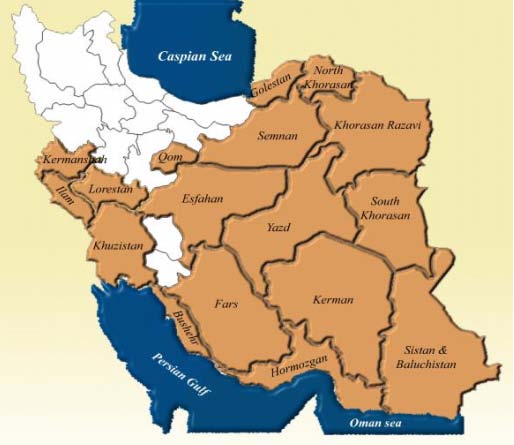
eishmaniasis is one of the most important communicable diseases between humans and animals transmitted to humans by sand fly species. The prevalence of ZCL in Iran has always been increasing, so that between 2001 and 2005 shows about a 105% increase. The known rural foci of Leishmaniasis have been reported from the villages of East Isfahan, Turkmen Sahara, Natanz, Sarakhs, Lotfabad, Khuzestan, Ilam, Khorasan, Shiraz, and Kashan [1][2][3][4][5] (Fig. 1). Due to the widespread prevalence of ZCL in Iran and the world, to break the disease transmission chain, appropriate practical approaches are needed, such as the use of various personal protection methods like long-lasting bed nets and insecticide-impregnated curtains, using insect repellents at work, and outdoors, indoor spraying is limited in scale 6 . As part of control programs, sand flies have been exposed to four major classes of synthetic insecticides: Organochlorine, pyrethroids, Organophosphates, and Carbamates. These exposures have been either intentional in directed vector control efforts or have been inadvertent as part of malaria control efforts against Anopheles 7 . The prevalence of insecticide resistance in vector species worldwide is a continuous threat for any success at mitigating the spread of vector-borne diseases. Most species of phlebotomine sandflies remain susceptible to insecticides. However, around the world, there is increasing evidence of insecticide resistance.
2. L
3. a) Detecting Insecticide Resistance
Managing insecticide resistance requires timely, accurate data through resistance monitoring and insecticide evaluation to assess a vector species' susceptibility to insecticides. The primary way to assess insecticide resistance in many vectors, including sand flies, is to use insecticide susceptibility bioassays. The two most commonly used bioassays worldwide are the WHO exposure kit bioassay and the Centers for Disease Control (CDC) bottle bioassay 8 . The WHO exposure kit bioassay is a standardized protocol that consists of an exposure kit containing tubes lined with filter papers that are impregnated with a specific concentration of an insecticide. The CDC bottle bioassay protocol consists of exposing insects to concentrations of insecticide that are coated on the interior of glass bottles. Both bioassays have been used to assess insecticide resistance in sand flies, but the WHO bioassay is used more frequently 7 .
4. b) Resistance Mechanisms
Insecticide resistance to synthetic insecticides have been reported in many important insect vectors such as mosquitoes, black flies, Triatomine bugs, lice, fleas, and sand flies. Four mechanisms of resistance are known to exist in insects: reduced penetration, behavior avoidance, target-site insensitivity, and metabolic detoxification. Of the four, target-site insensitivity and metabolic detoxification are the two most geographically and entomologically widespread. Today, there is evidence of target-site insensitivity and metabolic detoxification resistance to the four main classes of synthetic insecticides in all major vector species 7 . The insecticide resistance mechanisms in Ph. papatasi have not been identified, unlike the mechanisms of more intensely studied insects such as mosquitoes and house flies. Numerous susceptibility tests have been carried out in the foci of ZCL in Iran in against Ph. papatasi. This results are the reviews on the monitoring and mapping of insecticide resistance in Ph. papatasi in Iran.
5. c) Characteristics of Ph.papatasi
Sandflies are tiny insects, 1.5-3.5 mm in length, with a hairy appearance, large black eyes, and long, stilt-like legs. Sandflies can be distinguished from other Diptera, especially members of the Psychodidae family to which these insects belong, by the way, they rest their wings, which look like a V. The Sand-fly Ph. papatasiis the well-known vector of zoonotic cutaneous leishmaniasis and sand-fly fever 3 (2). This species is endemic to most parts of Iran 3 . The Ph.papatasi prefers human habitats rather than other spices even though it found in human habitats in mountainous areas 9 (3). Their Resting places are animal and human habitats also that caught from the plains place much more than the mountains. It is found in rodents' nests, rooms, stables, and wall crevices, and in all biotopes. It is interested in heat and humidity as well as this grows well where the groundwater level is high. This species is sensitive to heat but is resistant to rain. In terms of blood-feeding, it is more interested in human, rodent blood and bites several times during feeding to supplement its food [10][11][12] .
6. d) Distribution of Ph.papatasi in Iran
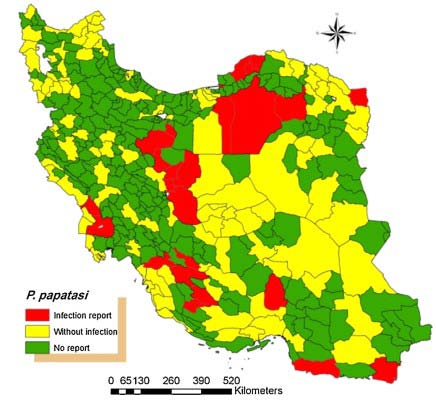
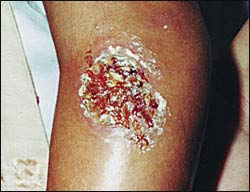
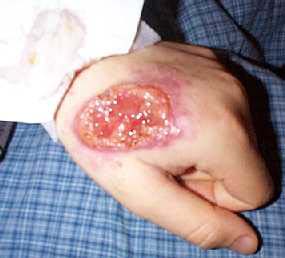
The sand fly Ph. Papatasi is widely distributed in Iran. There are in East Azerbaijan, West Azerbaijan, Ardabil, Isfahan, Ilam, Bushehr, Tehran, Chaharmahal Bakhtiari, Khorasan, Khuzestan, Zanjan, Semnan, Sistan and Baluchestan, Fars, Qom, Kurdistan, Kerman, Kermanshah, Golestan, Gilan, Lorestan, Mazandaran, Markazi, Hormozgan, Hamedan and Yazd 1,3,14 (Fig. 2). Figure 3 shows the symptoms of ZCL.
7. papatasi in Iran
The main vector of the rural type of leishmaniasis is Ph. papatasi, which is semi-wild. The most of transmission takes place in an outdoor place, so spraying does not have a significant effect on reducing cases except in the event of epidemics, which may be effective. Although the make use of bed nets, curtains treated by the deltamethrin insecticide with a shelf life of more than five years may lead to the severance of the transmission chain in rural seekers, the covered population must have been properly trained beforehand. Control of sandflies was started using residual insecticides such as DDT, lindane, and aerosols. DDT and lindane were used as emulsions, aqueous suspensions, soapy water suspensions, solutions, and powders. DDT in the form of aerosol was also very effective. DDT and lindane aerosols were mainly used for surface spraying. These compounds were characterized by their lasting effect. Sprayed surfaces retained their insecticidal effect for several weeks and even months after application.
8. II.
9. Method
To provide authentic information about these novel results, we used reliable data on academic resources such as Google Scholar, Scopus, Web of Science, Springer, Pro-Quest, Wiley Online, Science Direct, Research Gate, PubMed, Sage, and SID.
10. III.
11. Result and Discussion
A glance at the table number 1 and Fig. 4 provided reveals the susceptibility status of Ph. papatasi to DDT (4%), permethrin (0.75%), deltamethrin (0.1%), cyfluthrin (0.15%) and lambda-cyhalothrin (0.05%), In four different years 2011, 2013, 2017 and 2020. It has been estimated in the rural district of Badrood, Natanz County, Esfahan province. The results revealed that this species was resistant candidate to DDT but susceptible to other insecticides 13,[15][16][17] . In a similar study in Lorestan Province-Pol-e Dokh-tar, Rumeshgan, and Kuhdasht districts the results showed that this species was resistant to DDT 4% but susceptible to bendiocarb 0.1%, permethrin 0.75%, deltamethrin 0.05%, and cyfluthrin 0.15% [18][19][20][21][22][23] .
12. Conclusion
Constant monitoring, having a map of insecticide resistance in Iran can be alert for the health system and is a good guide for vector disease control. Furthermore, guidelines is needed for monitoring and evaluation of insecticide susceptibility tests against sand flies.
Declarations: All the author declare that there is no conflict of interest.
Statements on the authors' contributions: All the authors were involved.
| Area | Method | Insecticide | Susceptibility status | Ref. | year |
| Dehbakri County, | Aspirator | DDT4% | 100 | (4) | 2011 |
| Esfahan | Deltamethrin 0.05% | 100 | |||
| Lorestan Province | Hand | DDT 4% | R(87.7) | (5) | 2020 |
| catch/indoor/baite | S(92.0) | ||||
| d traps/outdoor | Bendiocarb 0.1% | S(93.4) | |||
| S(94) | |||||
| Permethrin 0.75% | S(92.4) | ||||
| S(97.9) | |||||
| Deltamethrin 0.05% | S (96.8) | ||||
| S (97.8) | |||||
| Cyfluthrin 0.15% | 100 | ||||
| 100 | |||||
| Arsanjan -Fars province | Aspirator | DDT4% | S (96.7) | (6) | 2000 |
| Natanz county, Esfahan | Aspirator | Deltamethrin 0.05% | S (97.86) | (7) | 2017 |
| ?-cyhalothrin 0.05% | S (97.78) | ||||
| Cyfluthrin 0.15% | S (100 ) | ||||
| Permethrin 0.75% | S (98.7) | ||||
| DDT4% | RC (96.) | ||||
| Natanz county, Esfahan | Aspirator | DDT 4% | Female, LT50:1312.66 | (8) | 2013 |
| Male, LT50:1200.97 | |||||
| Permethrin 0.75% | Female, LT50:2 | ||||
| 53.66 | |||||
| Male, LT50:310.10 | |||||
| Deltamethrin 0.1% | Female, LT50:36.47 | ||||
| Male, LT50:18.63 | |||||
| Cyfluthrin 0.15% | Female, LT50:9.36 | ||||
| Male, LT50:6.08 | |||||
| ?-cyhalothrin 0.05% | Female, LT50:6 | ||||
| Male, LT50 : 0.77 | |||||
| Natanz county, Esfahan | Aspirator | DDT 4% | Female, LT50:1104.97 | (9) | 2011 |