1.
. Phytochemicals have therefore provided the best alternative method for disease treatment and management (Oyelese et al., 2020). It was discovered long ago that some plant materials exhibit antibacterial properties. Recently, there is a growing demand globally by consumers in minimizing artificial preservation that can be detrimental to human health. Consequently, spices, herbs and naturally occurring phenolics from various plants sources are being studied in detail in response to consumer requirements for fresher and more natural additive-free products (Asif, 2015;Lourenco et al., 2019; Ulewicz-Magulska and Wesolowski, 2019; Adesina et al., 2021). Plants derives medicines are of immense benefits since they are relatively safer than synthetic alternatives, they offer profound therapeutic benefits and are more affordable source of treatment (Atanasov et al., 2015;Anand et al., 2019;Ololade et al., 2021). Plant based antimicrobials therefore represent a vast untapped source of medicines.
Tetrapleura tetraptera (Schumach. and Thonn Taub) is from the family of Mimosaceae and commonly known as "Aridan" in Nigeria. The medicinal plant is a perennial tree with dark green leaves and thick, woody base and spreading branches. The fruit consist of a fleshy pulp with small, brownish-black seed. The fruit possess a fragrant characteristic pungent, aromatic odour and flavour which has been attributed to insect repellent property (Odesanmi et al., 2010;Atawodi et al., 2014;Nwoba, 2015;Ozaslan et al., 2016;Larbie et al., 2020;Otimanam et al., 2020). Medicinally, the fruit is used to prepare soup or porridge for nursing mothers from the beginning of childbirth to prevent post-partum contraction, gastro-intestinal disorders especially stomach ulceration and to aid lactation in nursing mothers (Mpody et al., 2019). It has also been harnessed in the management of convulsions, leprosy, inflammation, flatulence, jaundice, malaria, rheumatism onset of diabetes mellitus in adults and as a molluscide (Uyoh et al, 2013).
2. Materials and Methods
3. a) Collection of plant material
The fruit samples were randomly obtained from Ota, Nigeria and identified by botanists as Tetrapleura tetraptera in the Department of Biological Science, Bells University of Technology, Ota, Ogun State, Nigeria.
4. b) Sample Preparation and Extraction
The fresh fruit pods of T. tetraptera were air dried and stored in air tight containers until required for use. The pods were cut into small sized pieces before pulverization using laboratory mortar and pestle and finally into powder with an electric blender. Pulverised sample was weighed with an analytical balance, 30 g were soaked in methanol, hot water and cold water respectively for three days with intermittent shaking. The extracts solutions were filtered and then concentrated using water bath (Ololade and Abiose, 2019).
5. c) GC-MS Phytochemical Screening of the Fruit Extract of T. tetraptera
The methanolic extract of T. tetraptera fruit was analysed using Shimadzu GC-MS-QP2010 Plus (Japan). The separations were carried out using a Restek Rtx-5MS fused silica capillary column (5%-diphenyl-95%dimethylpolysiloxane) of 30 m× 0.25 mm internal diameter (di) and 0.25 mm in film thickness. The conditions for analysis were set as follows; column oven temperature was programmed from 60-280°C (temperature at 60°C was held for 1.0 min, raised to 180 °C for 3 min and then finally to 280 °C held for 2 min); injection mode, Split ratio 41.6; injection temperature, 250 °C; flow control mode, linear velocity (36.2 cm/sec); purge flow 3.0 ml/min; pressure, 56.2 kPa; helium was the carrier gas with total flow rate 45.0 ml/min; column flow rate, 0.99 ml/min; ion source temperature, 200 °C; interface temperature, 250 °C; solvent cut time, 3.0 min; start time 3.5 min; end time, 24.0 min; start m/z, 50 and end m/z, 700. Detector was operated in EI ionization mode of 70 eV. Components were identified by matching their mass spectra with those of the spectrometer data base using the NIST computer data bank, as well as by comparison of the fragmentation pattern with those reported in the literature.
6. d) Preparation of Extract Solution for Antimicrobial Test
Stock solutions of the concentrated (methanol, hot and cold) fruit extracts (2.5mg/ml, 2.0mg/ml, 1.5mg/ml, 1.0mg/ml, and 0.5mg/ml) were prepared in dimethyl sulfoxide (DMSO). The solutions were stored in the refrigerator until time for use (Alao et al., 2018).
7. e) Antimicrobial Assay
Collection of isolates: Uropathogenic organisms which were identified as Staphylococcus aureus, Staphylococcus saprophyticus, Escherichia coli, Enterococcus faecalis and Pseudomonas aeruginosa were obtained from the stock collection of the Microbiology Laboratory of Bells University of Technology Ota, Nigeria. Stock solutions of the concentrated (methanol, hot and cold) fruit extracts (2.5mg/ml, 2.0mg/ml, 1.5mg/ml, 1.0mg/ml, and 0.5mg/ml) were prepared in dimethyl sulfoxide (DMSO). The solutions were stored in the refrigerator until time for use (Alao et al., 2018). In vitro antibacterial potential of the crude extracts were evaluated using agar well diffusion method.
Antibiotic Susceptibility Test: Antibiotic susceptibility test was carried out on each of the pathogenic isolates to determine their susceptibility to the conventional antibiotic dics. Multi
8. Results and Discussion
9. a) Phytochemical Composition of the Fruit Extract of T. tetraptera
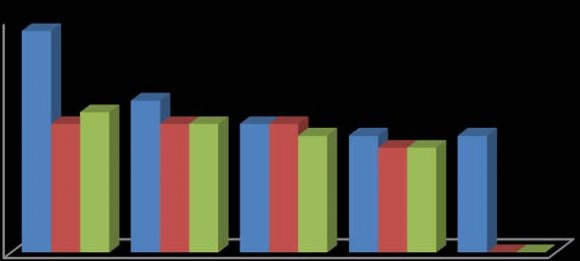
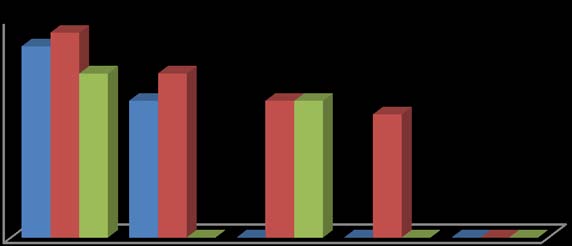
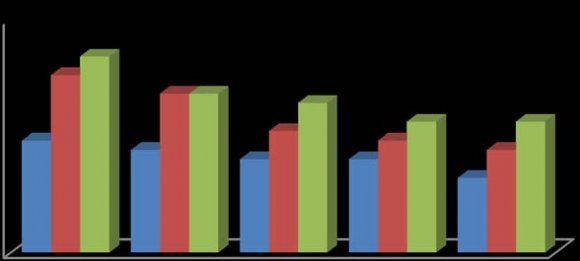
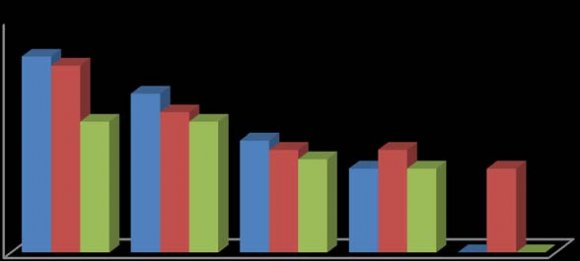
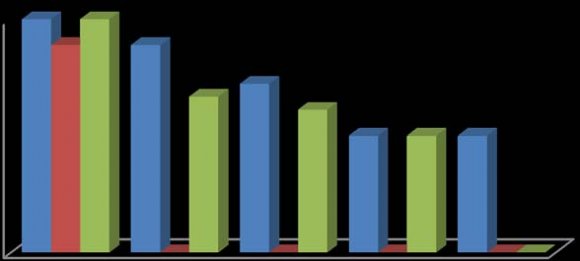
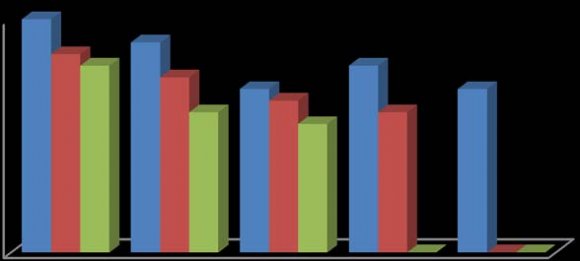
In this study, the fruit of T. tetraptera was investigated for its chemical constituents. The colours were dark green and brown, respectively. The concentrated extract was subjected to Gas Chromatography-Mass Spectrometry (GC-MS) analysis for detailed identification of its components. Identification of the compound was also aided by comparison of their GS-MS mass spectra database. The retention indices of each identified components were also calculated based on their retention time in order to confirm the identification. The GC-MS analysis of the fruit extract of T. tetraptera led to the identification of 35 constituents representing 96.28% of the extract. The compound, retention indices and percentage composition are given in Table 1, where the identified components were listed in order of their retention indices. The GC-MS analysis of the fruit extract of T. tetraptera led to the identification of 35 constituents representing 96.28% of the extract. Alletone (16.9%), 3hydroxydihydro-2(3H)-furanone (10.0%), 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (9.3%), 5-Hydrxoymethylfurfural (9.0%), (4E)-4-methyl-4-hepten-3one (9.0%) and 2,3-dihydothiophene (4.5%) were the most abundant components in the fruit extract of T. tetraptera. These compounds contribute greatly to the antimicrobial effects of T. tetraptera. The above results showed that the fruit extract of the sample grown in Nigeria and other West African countries has various medicinally active compounds and properties that have been used to treat a great variety of human diseases such as convulsions, leprosy, inflammation, flatulence, jaundice, malaria, adult onset of diabetes mellitus, In this study, different concentrations of the methanolic, hot water and cold water extracts of the fruit of T. tetraptera (2.5, 2.0, 1.5, 1.0, 0.5 mg/ml) were prepared) and tested on six pathogens (Staphylococcus aureus, Staphylococcus saprophyticus, Enterococcus faecalis, Serratia marcescens, Proteus mirabilis and Pseudomonas aeruginosa). Inhibition zones were observed for the tested organisms. The results obtained for each organism were shown in figure 1-6. Antibiotic sensitivity and resistance patterns of the isolates to standard antibiotic disc were shown in table 2. In this study, the leaves and fruit of this plant were used to determine the antimicrobial activity. The plant extracts were prepared using methanol, hot water and cold water by solvent extraction procedures and their antimicrobial properties were assessed using agar well diffusion method. The sample exhibited antibacterial properties against Gram positive and Gram negative organisms. The methanol extract showed the highest inhibitory effect. Then, hot water and cold water had similar inhibitory effects. The fruit had similar zone of inhibition ranging from 8-21 mm. However, fruit extract had wider range of activity at different concentrations. P. aeruginosa showed the highest zone of inhibition among the tested bacteria with the fruit extract was with a maximum zone of inhibition of 20 mm. For Staphylococcus aureus, the highest inhibitory effect was observed in methanol extract as depicted in figure 1. This ranged between 10-19 mm at various concentrations used in this study.
10. C
For Enterococcus faecalis, the highest inhibitory effect was observed in cold water extract, followed by hot water extract and least by the methanol extract as shown in figure 3. This was ranged between 08-21 mm at various concentrations used in this study. For Proteus mirabilis, the highest inhibitory effect was observed in methanol extract and cold water extract and then hot water extract did not show activity except at 2.5 mg/ml as shown in figure 5. The value of zones of inhibition was ranged between 09-18 mm at various concentrations considered in this study. For Pseudomonas aeruginosa, the highest inhibitory effect was observed in methanol extract followed by hot water extract and then cold water extract as shown in figure 6. The value of zones of inhibition was ranged between 11-20 mm at various concentrations considered in this study. In addition, the effect observed was dependent on the concentration of the extracts and the extract established an interaction with the concentration used as the range of activity reduced with the decrease in concentration of each extraction solvent. Finally, the effects measured was also dependent on the extraction method and solvent (absolute methanol, hot water and cold water) used and the fruit established an interaction with the extraction method. Table 2 showed the susceptibility of the tested organisms to different antibiotics. All of them were inhibited by at least one antibiotic with no exception. They were all resistant to Augmentin, Ceftazidime, Cefuroxime, and Cloxacillin. Also, the findings from this study indicated higher resistance pattern exhibited by the organism to synthetic antibiotic in comparison to the high inhibitory effects of T. tetraptera extracts against these organism. Therefore, if the plant can be adequately harnessed and studied, it can be used as a natural antibacterial agent against some of the pathogens as discovered in this study.
Solvent polarities are factors that responsible for the variation in the antibacterial activity of plant extracts, permeability of cell of bacteria, concentration etc (Gonelimali et al., 2018;Zhang et al., 2020). The effect of solvent polarity on extraction yield and antibacterial properties of secondary metabolites in the fruit was studied. Solvent type and polarity index play an important role in the antibacterial activities level in the IV.
11. Conclusion
This study revealed that the fruit extract of T. tetraptera commonly used by the local people in Africa in the preparation of herbs, has the potential of being used in the production of drugs with a broad spectrum of activity. This study also serves as an affirmation that the traditional application of sample is of great essence and that it possess antimicrobial properties which can be used for the treatment of a wide range of diseases. The antimicrobial activities of T. tetraptera was investigated in this study and proven that it is a potential source of antibiotics for the development of newer and more effective antibacterial agent. With respect to this study, it is recommended that clinical studies should be carried out on this plant to harness its potential for drug production.
12. Conflict of Interest:
We have no conflict of interest.
| -sensitivity discs bearing eight | |||
| different | antibiotics | Augmentin, | Ceftazidime, |
| Cefuroxime, III. | |||
| Compound | Retention Index | % Composition |
| 3-methyl-4-(phenylthio)-2-prop-2-enyl-2,5- | 0 | 0.6 |
| dihydrothiophene | ||
| 3-methyl-2-heptanol | 130 | 1.6 |
| sec-butyl nitrite | 544 | 0.2 |
| tutane | 598 | 1.0 |
| sec-butylamine | 598 | 1.0 |
| N-methylisobutylamine | 653 | 1.0 |
| 2,3-dihydothiophene | 723 | 4.5 |
| 3-methyl-3-ethylpentane | 732 | 2.5 |
| N-methyl-N-(4-pentenyl)amine | 806 | 1.0 |
| propylene Carbonate | 875 | 0.2 |
| pimelic ketone | 891 | 6.3 |
| (4E)-4-methyl-4-hepten-3-one | 938 | 9.0 |
| 3-hydroxydihydro-2(3H)-furanone | 1013 | 10.0 |
| alletone | 1022 | 16.9 |
| 1,3-butylene glycol diacetate | 1087 | 0.03 |
| octylmegthylamine | 1114 | 1.0 |
| 5-hydrxoymethylfurfural | 1163 | 9.0 |
| (+/-)-citronellol | 1179 | 0.03 |
| 3,5-dihydroxy-6-methyl-2,3-dihydro-4H- | 1269 | 9.3 |
| pyran-4-one | ||
| (2E)-2-undecenyl acetate | 1489 | 0.03 |
| decane-1, 10-diol | 1501 | 13.0 |
| 1-ethyldecyl acetate | 1516 | 0.03 |
| D-glucitol, 1,4:3,6-dianhydro-, dinitrate | 1678 | 0.2 |
| myristic acid | 1769 | 3.2 |
| methyl 14-methylpentadecanoate | 1814 | 0.1 |
| palmitic acid, methyl ester | 1878 | 0.1 |
| methyl 15-methylhexadecanoate | 1914 | 0.1 |
| palmitic acid | 1968 | 3.2 |
| phytol | 2045 | 0.03 |
| trans-phytol | 2045 | 0.03 |
| methyl elaidate | 2085 | 0.1 |
| methyl (10E)-10-octadecanoate | 2085 | 0.1 |
| methyl cis-octadec-11-enoate | 2085 | 0.1 |
| linolelaidic acid, methyl ester | 2093 | 0.6 |
| 1,4-diacetyl-3-acetoxymethyl-2,5- | 2105 | 0.2 |
| methylene-1-rhamnitol | ||
| Percentage Total | 96.28 | |
| © 2022 Global Journals |
| Isolates | OFL 5?g | AUG 30?g | CAZ 30?g | CRX 30?g | GEN 10?g | CTR 30?g | ERY 15?g | CXC 5?g |
| E. faecalis | 34 | R | R | R | 21 | 12 | R | R |
| P. aeruginosa | 21 | R | R | R | 15 | 26 | R | R |
| S. marcescens | 22 | R | R | R | 16 | 23 | R | R |
| S. saprophyticus | 16 | R | R | R | 15 | 15 | R | R |
| P. mirabilis | 21 | R | R | R | 15 | 10 | R | R |
| K. pneumoniae | 15 | R | R | R | 15 | R | 15 | R |
| S. aureus | 28 | R | R | R | R | R | R | R |