1. I. Introduction
yclin-dependent kinases (CDKs) are group of protein kinases (serine/threonine kinases), activated via formation of a complex with cyclin molecules, involved in cell cycle regulation. CDKs are considered as potential target molecules for anti-cancer medication. The level of CDK remains constant in a cell, while cyclin level fluctuates depending upon cell cycle stage. It has been reported that each cyclin is associated with one or two CDKs and most of the CDKs get associated with one or two cyclin molecules. Cyclin-CDK complex formation results into activation of CDK active site. Formation of this complex is regulated via various phosphate and kinase molecules including CDK-activating kinase (CAK), Cdc25 and Wee 1 kinase (1). CDK also get activated via non-cyclin CDK activators such as CDK5 Activators, Viral Cyclins and RINGO/Speedy (2,3). Some of the alternative names for CDK include cell division protein kinase 1, Cell division control protein 2 homolog and p34 protein kinase (4). CDKs have been categorized into CDK1 / CDC2, CDK2, CDK3, CDK4, CDK5, CDK5R1, CDK7, CDK8, CDK9 / CDC2L4, CDK16 / PCTAIRE1, CDKL2, CDKL3, CDKL4, CDKL5 (1). The phosphorylation at threonine-14 or tyrosine-15 causes inactivation or deregulation of its enzymatic potential while phosphorylation at threonine-161 around the T-loop activates it (5). The CDK 7 member acts indirectly, by acting as CDK-activating kinase (CAK) that cause phosphorylation of other CDKs (especially CDK1, CDK2, CDK4, and CDK6 molecules) (6).
Cyclin-dependent kinase activity requires phosphorylation at active site of threonine residue. The phosphorylation time frame varies across model organisms. It has been reported in mammalian cells that the activating phosphorylation take place after cyclin binding; while in yeast cells, the activating phosphorylation usually occurs before cyclin binding. The activity of CDK kinase is not regulated via known cell-cycle pathways. It has been reported that cyclin binding is actually a limiting step for CDK activation (6). The CDK activating kinase is usually composed of CDK7, cyclin H and Mat1assembly protein. Phosphorylation of activation segment is not prerequisite for CDK7/cyclin H complex activation in presence of Mat1; while in absence of Mat1, phosphorylation at Ser170 and Thr176 in the activation segment of CDK7 is required for its activity. It has been self phosphorylates, but have ability to tendency to phosphorylate each other (7). Morgan (2007) laevis is also recognized as M015. It has been reported
that CAK activity remains high during cell cycle via unknown control mechanism. In G0 quiescent state CAK activity is comparatively low, compared to tumor cells (6). It is a matter of fact that CAK is localized to nucleus in many vertebrates. This phenomenon suggests that CAK is involved in transcription along with cell regulation. It has been reported that CDK7 (a type of CAK) is involved in phosphorylation of cellular transcriptional machinery (8,9). Serizawa et al, reported strong association of CDK-activating kinase subunits with transcription factor Transcription Factor IIH (TFIIH) which suggested their role in transcriptional regulation as well as in cell-cycle control (10). Shiekhattar et al, reported CAK complex as an important component of human transcription factor TFIIH, their findings suggested that phosphorylation of both Cdc2 and CDK2 creates link between cell cycle regulation and transcription (11).
2. II. Literature Search
A review of literature was conducted via accessing latest research articles from Pubmed, Google Scholar by using the key words: Cyclin-dependent kinases, CDK activating kinases, Cell cycle regulation via CDKs, Interactions of CDK activating kinase, Association of CDK activating enzymes with cellular proteins, Structure and Function of CDK activating kinases. Most relevant research articles of previous two decades were considered for review. The anatomical and biological context of Cak1 was kept into consideration and CAK1 related enzymatic, physical and regulatory interactions were contemplated. High impact information was pooled into three categories of "Association of CDK activating kinases (CAKs) with Cyclin-dependent kinase", "Structural characterization of CDK activating kinases (CAKs) activation" and "Functional characterization of CDK activating kinases: interactions with other cellular proteins".
3. a) Association of CDK-activating kinases (CAKs) with
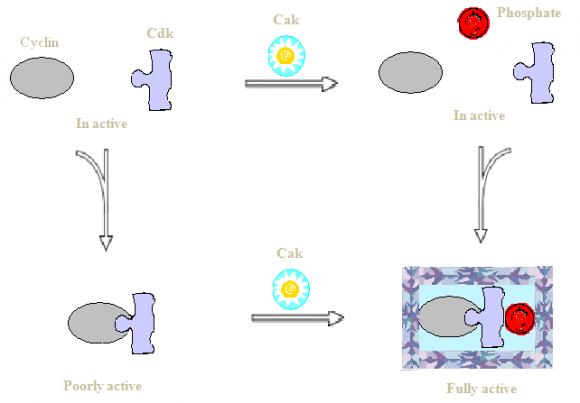
Cyclin-dependent kinase TFIIH was identified initially as basal transcription factor associated with transcription of protein-coding genes. The cloning of nine vital TFIIH subunits revealed its importance in repair of damaged DNA and cell cycle regulation (both of which are fundamental processes in cell). It is quite obvious that TFIIH is involved in various other cellular metabolic process, thus mutation in some of its subunits may cause serious human disorders leading towards complex pleiotropic symptoms such as susceptibility towards cancer, developmental abnormalities and UVlight sensitivity. The study conducted by Keriel et al discussed ternary subcomplex of TFIIH and its importance as CDK-activating kinase due to its tendency towards activating CDKs via phosphorylation along with its vital enzymatic activities of RNA synthesis and DNA repair (12). Nasmyth et al and Beach et al, reported a single CDK (Cdc28p or its ortholog Cdc2) was found responsible for all important cell cycle transitions (13,14). The CDK3, CDK4 and CDK6 are involved regulation of G1-S phase transition, whereas CDK2 is associated with entrance into S phase and replication of DNA; while CDK1 is vital for mitosis (15)(16)(17)(18)(19). The CDK activity is regulated in cells via four basic mechanisms; which includes, binding of cyclin proteins to get activated, inhibition of CDK activity via cyclindependent kinase inhibitors, conserved residues phosphorylation at ATP-binding pocket of CDK (for inhibition of its activity) and phosphorylation at a conserved residue of CDKs T-loop (for its activation) (20). Loss of CAK activity usually lead towards cell cycle arrest and transcriptional defects (21)(22)(23). Phosphorylation at conserved threonine residue of Tloop do not play a direct role during catalysis, instead it tends to stabilize CDK-cyclin complex (24)(25). Various model systems indicated that the phosphorylation may proceed independent to complex assembly, contrarily the assembly of complex may also occur before or after phosphorylation as shown in figure 1 (26).
In 1996, while working on S. cerevisiae, studies conducted by Espinoza et al, Kaldis et al and Thuret et al elaborated identification of novel CAK protein. The CAK (CAK1/Civ1) enzyme of yeast was isolated and purified via assistance of biochemical fractionation. There exists a strong correlation between Cak1 and Cdc28 (of budding yeast), as compared to rest of kinases. The Cdc28 usually lack consensus sequence of Gly-x-Gly-x-x-Gly in the ATP-binding loop (where X represents aminoacid). In CAK1 the aforementioned sequence is replaced by Asp-Ile-Thr-His-Cys-Gln. A 45 kDa purified bacterial CAK1 has tendency to phosphorylate both cyclin bound form of CDK2 and monomeric Cdc28 at invitro conditions. This ability indicates the ability of CAK1 to function in absence of regulatory subunit protein or post-translational modifications. The yeast cell extract may be used to purify both CAK1 along with Cdc28. Studies suggested antibodies, reduces CAK activity which clearly indicated vital role of CAK1. On the contrary, over expression of CAK1 in purified yeast extract yielded increased CAK activity (27,28,29). Although CAK1 is structurally more related to CDKs, yet there exists some dissimilarity. The CAK is unique in the sense that it exists as monomer during its functionally active form, and it also lacks the glycine-rich loop in its structure. It can phosphorylate Cdc28 monomer. The phosphorylated Cdc28 could get activated via addition of cyclin molecule which supported the indication that cyclin binding prior to CDK phosphorylation is not a necessary step. It can be inferred from the literature that; for catalysis, the phosphorylation and cyclin binding only tends to provide structural stability, which further illustrates that aforementioned events are not necessary steps for catalysis (30,31). A true homologue of CAK1 (of S.cerevisiae) exists in higher eukaryotes which are regulates CAK activity (26).
4. b) Structural Characterization of CDK activating kinases (CAKs) activation
The binding of cyclin molecule and CDK activating kinase to CDK2 leads towards important conformational changes at active site. Insights into ATP binding at active site revealed orientation of phosphate outwards, while substrate binding at active site cleft. During inactive state, CDK2 is unable to bind substrate molecule and gets disoriented ATP positioning. Inactive conformation causes PSTAIRE helix to move outwards via a L12 helix push as shown in figure 2. The disoriented ATP positioning is due to PSTAIRE helix disposition which carries glutamate 51 residue (vital for positioning ATP phosphates) (6,9). During activation state, conformational changes appear after cyclin A binding to the molecule. At this state, the T-loop displace from entrance point of active site thereby reducing blockage of substrate binding site. Active conformation causes PSTAIRE helix to move inside along with L12 helix rearrangement as beta strand, which results into glutamate 51 interaction with lysine 33 residue. During this state, there occurs to be repositioning of Aspartate 145. Aforementioned structural modifications and rearrangements results into most appropriate binding of ATP phosphates. After phosphorylation of threonine 160 of CDK via CAK, the interactions between T-loop and cyclin A gets increased.
The event of phosphorylation increases stability and activity of cyclinA-CDK2 complex. It has been reported that different conformational changes appear in CDKs depending upon types of cyclin molecules. CAK exist as trimeric enzyme containing CDK7, Cyclin H and MAT1. CAK was unusually identified as 44 kDa CAK1 protein which resembled CDKs. The activity of CAK1 remained constant throughout cell cycle. The responsible gene (CAK1) was found essential for cell viability. The information revealed that there exist a difference among CAK of vertebrates and nonvertebrates which suggest distinct mechanisms of CDK activation among vertebrates and non-vertebrates (32). It has been reported that CDK7 is vital for mitosis and CDK activating kinase at invivo conditions. It was found that CDK7 is essential for Cdc2/cyclin A and Cdc2/Cyclin B complexes and cell division (33). Schindler et al, reported that CDK activating kinase,
2 50CAK1p is involved in activation of meiotic S phase via Ime2p. There are many Cdc28 independent functions of CAK1 which are unique with respect to meiosis. An example of such functions is to induce S phase, whose regulation is different in both mitosis and meiosis. During mitosis, Cdc28 protein usually controls its Sphase promoting ability via destroying its inhibitor through signaling event. During meiosis, the Ime2p protein kinase induces signaling which causes Sic1 destruction. It was found that it is CAK1 which is involved in Ime2p activation, which suggests Ime2p as potent target for CAK1p regulation (34).
It has been reported that CAK1p nucleotide binding pocket is significantly different from other protein kinase molecules which suggest importance of specific target molecule as inhibitory drug. The 5`fluorosulfonylbenzoyladenosine (which as an ATP analog) usually inhibit protein kinases, but its activity has been found insensitive towards CAK1p (35). Yao et al reported CAK1 as physiological regulator of Bur1 kninase. This indicates that activation of Bur1-Bur2 cyclin dependent kinase complex is dependent upon CAK1 (36). CAK1 is involved in Ctk1 C-terminal domain phosphorylation at Thr-338. Invitro study revealed that CAK1 directly phosphorylates Ctk1 in S. cerevisiae (37).
Espinoza et al reported that CAK1 is required for Kin28 phosphorlyation and invivo activation of Cdc28 (38). Immunofluorescence and biochemical subcellular fractionation techniques have confirmed that CAK1p is completely dispersed in cell. It has been reported that CAK1p level is usually stable during growth phase or stationary phase, while its level fluctuates during meiosis. This phenomenon depicts CAK1p regulation at both transcriptional and post transcriptional level (39).
The CAK usually exist as "free CAK" and "associated CAK". Quantitatively, free CAK is predominant as compared to associated CAK. The "free CAKs" are involved in phosphorylating CDKs, which controls cell cycle regulation. The "associated CAKs" are associated with transcription factor TFIIH. These CAKs are involved in phosphorylating transcriptional proteins (such as RNA polymerase II). The CAK molecule is also involved in promoter clearance and transcription (from pre-initiation to the initiation stage). CAK are also involved in enhancing transcription rate by phosphorylating estrogen receptors and retinoic acid which leads towards increased expression of target genes. CAK plays a vital role in DNA damage response and CAK inhibition usually prevents cell cycle progression (9).
5. III. Conclusion
Studies depicted that increased activation of certain cellular proteins may causes pathogenesis of tumor formation and cancer propagation while elevated activation of such proteins can be inhibited via ATP and other potential inhibitors to cure associated cancers (40,41). The CDK activating kinase is an important cell cycle regulating molecule. Cancer associated cell cycle defects are frequently mediated through alterations in CDK activity. Research suggests that the tumor cells require specific interphase CDKs for abnormal proliferation, therefore inhibition of CDK and CDK activating kinases could provide potential therapeutic target against human neoplasias.
6. IV. Acknowledgment
We would like to thank Sameen Ruqia for providing technical support during data management.

| cerevisiae, S. pombe, D. melanogaster, X. laevis and H. |
| sapiens. S. cerevisiae possesses CAKs including CAK1 |
| (also known as Civ1) and Kin. The CAK 1 are monomer |
| with non cyclin partner, while Kin 28 are CDK7 related |
| with no CAK activity. S. pombe possesses CAKs |
| including Csk1 and Mcs 6. The Csk1 is monomer and |
| related to Cak1 while Mcs6 is related to CDK7 and |
| usually binds to cyclin Mcs2. D. melanogaster, X. laevis |
| and H. sapiens possesses CDK7 as CAK that forms |
| trimer with cyclin H and Mat1. The CAK (CDK1) of X. |