1.
Ovitrap Surveillance of Aedes Mosquitoes (Diptera: Culicidae) in Selected Areas of Dehradun District, Uttarakhand, India N. Pemola Devi ? , R.K. Jauhari ? & Ritwik Mondal Abstract-Background & Objective : Dengue, a major public health problem in India is caused mainly by Aedes aegypti and Ae. albopictus. In Uttarakhand State (India), there has been a heavy increase in dengue cases in the year 2010 and thereafter in 2011-12, there was a decline. Keeping in view a change in climatic scenario i.e., heavy rainfall during June to September, we are expecting more and more cases of dengue this year too. Since there is lack of information on the bionomics of the recognized vectors of dengue from this region, it has been planned to determine the efficacy of ovitraps in monitoring the distribution and abundance of Aedes species in different urban and suburban areas of district Dehradun, Uttarakhand.
Result: As many as 6 species of Aedes viz., Aedes aegypti, Ae. albopictus, Ae. edwardsi, Ae. pseudotaeniatus, Ae. unilineatus and Ae. vitattus were collected during the study period. Ae. aegypti shared highest (37.28%) followed by Ae. albopictus (33.27%), Ae. pseudotaeniatus, (15.68%) and Ae. vitattus (8.33%). The mixed breeding comprised larvae of Culex, Anopheles and some unidentified species and shared least percentage (3.10%). In indoor, overall mosquito accounts low percentage (18.82%) in all three localities as compared to outdoor percentage (19.47%). Maximum ovitrap index was encountered from Garhi Cantt. (48.75) followed by Karanpur (45.00) and Sahastradhara (43.75) during August 2012. Outdoor indices of area ovitraps index were in the range of 17.30±1.83 to 21.88±2.10, while in indoors the range was 12.30±1.67 to 15.42±1.56. Monthy ovitrap index of the study period ranged from 0.00 to 45.83.
2. Introduction
engue, a major public health problem in India is an arbo-viral disease caused by the dengue virus (DENV) (Family: Flaviviridae) comprising four serotypes (DEN-1, DEN-2, DEN-3 and DEN-4) and female Aedes mosquito, mainly Ae. aegypti and Ae. albopictus play a role in the transmission of disease. Dengue Virus Infection (DVI) cause a spectrum of disease ranging from mild infection (dengue fever, DF) to a severe deadly disease -dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS) [1]. About 40% of the global population is living in the areas where transmission of dengue occurs. In an estimate, 50 million dengue infections, including 5,00,000 cases of DHF require hospitalization every year [2]. Earlier, estimated 3.46-3.61 billion people live in areas at risk of dengue from 124 countries which correspond to 53.0-55.0% of the world population [3]. Due to global warming Ae. aegypti and Ae. albopictus moved northward and had more rapid metamorphosis, the WHO expects millions more to be affected in the coming years [4].
In district Dehradun, the Dengue infections are well established from the year 2006 onwards. The abundance of vectors species have been reported by earlier works [5,6] who observed the breeding of Ae. aegypti in both natural habitats and domestic containers. Larval population of Ae. aegypti has been recorded in drains, pits, streams, canals, containers and tree holes while the breeding of Ae. albopictus was recorded in tanks, ponds, streams, containers and tree holes from district Dehradun [7]. The occurrence of Ae. vitattus in Garhwal region in Uttarakhand state was recorded in the past [8,9] and in recent years too [10]. Moreover, from Nainital district in Kumaon region of Uttarakhand, entomological investigations during an outbreak of Dengue fever in Lal Kuan town revealed larval and adult stages of Ae. aegypti and Ae. albopictus in transmission season [11].
Ae. aegypti is an urban mosquito that breed almost entirely in man-made containers (cistern, flower pots, tanks, tyres and cans) found in and around households, construction sites, factories etc. On the contrary, Ae. albopictus breeds in both man-made containers as well as in natural containers such as bamboo, tree holes and leaf axils. Ovitrap surveillance is the most common sampling method to monitor Ae. aegypti and Ae. albopictus populations through their egg laying activities [12]. It has been claimed to be a more effective and sensitive technique as compared to the conventional larval surveys, especially when the Aedes infestation rates were very low [13].
Keeping in view that for the last 3-4 years, on one hand there is an increase in Dengue cases in Dehradun (India) while on the other hand, lack of information in bionomics of Aedes sp. involved in Dengue transmission, it was decided to determine the efficacy of ovitraps in monitoring the distribution and abundance of Aedes species in different urban and suburban areas of district Dehradun in Uttarakhand state, located in the northern India.
3. II.
4. Methods a) Study Area
The present study was carried out mainly in urban area of Doon Valley (latitude 30 o 19'N, 78 o 04'E, longitude 77°35'E to 78°20'E) in district Dehradun (Uttarakhand). Ovitrap surveillance was conducted at three sites of Dehradun city: Sahastradhara, Garhi Cantt. and Karanpur from August 2012 -July 2013. The ecological description of the study sites is being provided as under -
5. b) Ovitrap surveillance
Each ovitrap was placed indoor and outdoor in randomly selected houses scattered over the study area. The paddles were collected individually from the ovitraps on weekly basis. Thereafter, fresh paddles were put in the ovitraps jar and the water level was adjusted so that they would remain moist. Collected paddles were submerged into a bowl of water containing larval food. The hatched larvae were subsequently counted and reared in the cages to emerge into adults. The adults were identified upto species level using respective Keys and Catalogues [14,15,16]. The estimation of ovitrap indices was done following the protocols developed [17,18]. The following indices were work out:i. Ovitrap Index (OI): The percentage of Aedes positive trap. ii. Area Ovitrap Index (AOI): Calculating the extensiveness of the distribution of the Aedes mosquitoes in a particular area.
iii. Monthly Ovitrap Index (MOI): Monthwise Aedes positive trap (average of all AOIs).
III.
6. Results
During the study period, 20 ovitraps (10 indoors and 10 outdoors) were installed for each week in each locality and observed the ovitrap index on monthly basis (Table 2). Maximum index was encountered from Garhi Cantt (48.75) followed by Karanpur (45.00) and Sahastradhara (43.75) during the month of August. During January and February, the breeding index was found nil. In all selected localities, the ovitrap indices were high during June to September. The mean indices were 18.65, 15.63 and 15.94 at Sahastradhara, Garhi Cantt and Karanpur respectively.
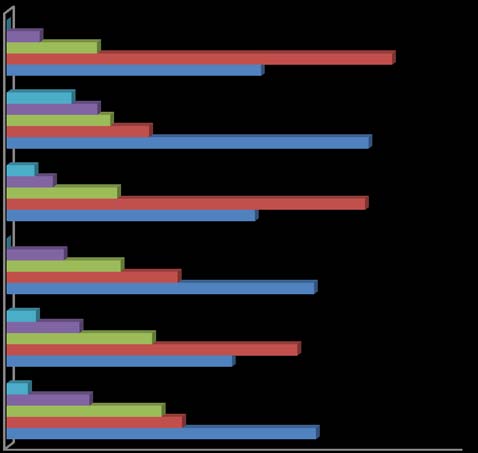
Fig. 1 shows the Area Ovitrap Index (AOI) of the selected sites during the study period. The outdoor indices were in the range of 17.30±1.83 -21.88±2.10. Highest index was found at Sahastradhara (21.88±2.10) followed by Karanpur (19.58±1.94) and Garhi Cantt (17.30±1.83). All the indoor ovitraps showed low index in all three localities in comparison to outdoor ovitraps (12.30±1.67-15.42±1.56).
Fig. 2 shows the MOI of the study period ranging from 0.00 to 45.83. Highest MOI was found during August (45.83) followed by July (36.66) and September (30.41). During the winter months like January and February, the index was recorded nil.
Fig. 3 shows the composition of Aedes mosquitoes in ovitraps at selected sites. A total of 6 Aedes species viz., Aedes aegypti, Ae. albopictus, Ae. edwardsi, Ae. pseudotaeniatus, Ae. unilineatus and Ae. vitattus. were collected. Of these, Ae. aegypti shared highest (37.28%) followed by Ae. albopictus (33.27%), Ae. pseudotaeniatus,(15.68%) and Ae. vitattus (8.33%). The mixed breeding comprising larvae of Culex, Anopheles and some unidentified species shared 3.10% only. In indoor, overall mosquito accounts low percentage (48.2%) in all three localities as compared to outdoor percentage (51.8%).
IV.
7. Discussion & Conculsion
Owing to inherent human behaviour and some traditional habits, detection of the presence of different mosquito vectors in urban situations has been a difficult task. It has been observed that the vector species are common in most areas on account of deficient water management, presence of non degradable and longlasting water holding containers and materials, as well as increasing urban agglomerations and inability or lack of mobilization to the population to the need to eliminate mosquito breeding sites. In a study conducted on dengue vector surveillance at Malaysia, the mosquito abundance was found related to population and human activity [19]. Occurrence of positive ovitraps in sampled houses positive ovitraps is an indication of human activity that provides a suitable environment for the propagation of these vector species in the residential area.
Earlier, it was stated that Ae. aegypti is strictly domiciliary, preferring less vegetation, biting indoors and primarily found indoors, while Ae. albopictus is found outdoors and breeds in all types of natural containers [20,21]. In these aspects, there is a bit similarity with the results of our study.
Dengue is a disease associated with the slum areas, where breeding of Aedes mosquitoes is most prevalent [22]. However, the ovitrap surveillance in the selected areas showed that Aedes mosquitoes are not only associated with the slum areas, but they are also associated with the residential area. As per the gathered observations, the settlement site had numerous natural and artificial containers providing good larval habitats. But the residential sites had a clean environment, with minimal natural containers. As all the houses had piped water supply, thus there was no necessity for the residents to store water. From our observations, the residential sites had minimal natural containers. The only possible habitat for Aedes mosquitoes was the concrete drainage system outside the houses. The drains had clear stagnant water with fallen leaves and other debris. Aedes larvae require clear, but not necessarily clean water and this was provided by the clear stagnant clear water of the drain [23,24]. In this way the drains served as good artificial larval containers for Ae. aegypti.
In the past, it was found out that Ae. aegypti rests in secluded locations inside homes such as under beds, in closets and on curtains [25]. In contrast, Ae. albopictus which breeds in both man-made containers such as cans, tires and water jars; as well as natural containers such as bamboo, bromeliads and coconut shells is more cosmopolitan in its feeding habitats and rests both inside and outside homes, making control difficult.
Aedes population has been observed in the ovitraps in both indoor and outdoor placement in urban residential sites, through the positivity of ovitraps was more in outdoor than indoor [26], thus resembling with our studies. Further, similar results were obtained in a study on surveillance of Aedes mosquitoes in a University Camps in Kuala Lumpur [27]. This may be due to availability of natural potential breeding sites such as bamboo tree, tree holes and mudden broken containers in outdoor environment.
Conclusively, the prevalence of a high density of dengue vectors in an urban area inspires an intensefication of the vector surveillance activities jointly with community participation.

| Study site | Ecological description |
| Sahastradhara | Abundant natural vegetation like trees and shrubs, |
| clean environment and mainly two-storied newly | |
| made buildings. | |
| Garhi Cantt. | Lush green vegetation, environment is clean and in |
| general newly made buildings exist. | |
| Karanpur | Less vegetation, environment partly clean and |
| highly populated and both old and new buildings | |
| are common. |