1. Introduction
xcretion is a process by which drug is removed from the site of action and eliminate from the body. The body begins to eliminate the drug by hepatic or renal metabolism or in some cases both, after administration of dose. The renal clearance of a substance is the volume of plasma that is completely cleared of a substance by the kidney per unit time. The kidneys are the primary mean for elimination waste products of metabolism that are no longer needed by the body. These products include urea, creatinine, uric acid and end product of hemoglobin breakdown and hormone metabolites. These waste products must be eliminated from the body as rapidly as they produced. The kidneys also eliminate most toxins and other foreign substances that are either produced by the body or ingested, such as pesticides, drugs and food additives. The two kidneys lie on the posterior wall of abdomen, outside the peritoneal cavity. Each kidney of adult human weights about 150 grams and is about in size of clenched fist. The medial side of each kidney contains an indented region called the helium through which pass the renal artery and vein, lymphatic's, nerve supply and ureter which carries the final urine from the kidney to the bladder, where it is stored until emptied (Guyton and Hall, 2000).
Renal clearance is quantity of fluid which is filtered out from blood through kidney or 'quantity of blood cleared in unit time' (Seldin, 2004). Allopurinol is structural analogue of xanthine oxidase which is enzyme for uric acid production. The active metabolite of allopurinol is oxipurinol which inhibit the xanthine oxidase. Xanthine oxidase converts xanthine and hypoxanthine into uric acid which increases from its limit and causes gout in humans. Allopurinol is taken through injection or in the form of tablet. If allopurinol is taken orally then after 1hour it achives high serum concentration and its bioavailability is about 67 to 90% withen this time period (Mathus et al, 2007). Allopurinol first convert into oxipurinol by the enzyme aldehyde oxidase and oxipurinol is metabolic product of allopurinol. In urine allopurinol remains unchanged about 10% of its total and about 70% is converted in the form of its metabolite oxipurinol form remaning 20% is excreted through feaces (Hande et al, 1984). The patients of renal disfunction should receive low concentration of allopurinol because increased oxipurinol concentration in plasma is very toxic for this type of patients (Perez et al, 2005). In case of renal impairment the allopurinol dose adjustment should be done by analyzing the creatinine clearance of that patient or it can be optimized by observing the serum concentration level of oxipurinol (Takada et al, 2005). Active metabolic product of allopurinol (oxipurinol) and allopurinol itself are the inhibitors of the enzyme xanthine oxidase which main function is the conversion of hypoxanthine into the xanthine and then xanthine into uric acid. Allopurinol mainly use in the management of long-lasting gout and the condition of hyperuricaemia linked with leukaemia. Allopurinol is a purine base isomer of significance as an anti gout agent. (Rodolfo and Juvencio 2003). Through the process of excretion allopurinol eliminated from the body and removed mostly through urine within certain time period. All metabolic products of allopurinol are removed through excretion. The kidney is a main organ for excretion of allopurinol in almost all mammals (Namazi, 2004). The purine base heterocyclic family of drugs and their basic equivalents are related in pharmacologic and biochemical procedures. From these heterocyclic allopurinol (pyrazolo [3,4-d] heart alongside harm due to free oxygen base radicals (ROS) on patients suffering heart bypass operation. But however the mechanism behind this protection is yet not well known. Allopurinol is a anti oxidizing drug that inhibit the xanthine oxidase enzyme necessary for uric acid synthesizing inside the body (Veller et al. 1994). The xanthine oxidase produces reactive oxidizing species in ischemic situation inside body which causes hyperuresemia and raises uric acid level and cause gout however a direct hunting ability of allopurinol and its metabolite oxipurinol alongside extremely oversensitive per oxidants as hydroxide free radical and hypochlorous acid radical. Allopurinol has showing to apply an inhibitory action counter to copper mediated ascorbic acid and DNA oxidation (Domenico et al. 1998). The allopurinol producing companies clearly demonstrate that therapeutic range of oxipuranol in human serum must be 5-15 mg/liter (Hande et al, 1998). If allopurinol and uricosauric drugs are coadministered then oxipuranol renal clearance increases. These drugs used for decreasing serum urate level by interaction with (URAT1) transporter (Iwanageer et al, 2005). The uricosauric drugs combination with allopurinol is only done in the condition of severe gout patients but whatever a condition the serum oxipurinol level should be optimized. The patient use allopurinol monitored by therapeutic drug monotring for verification of patient adherence for proper handling of complications (Stamp et al, 2000). Allopurinol lowers the uridine and uric acid concentrations in plasma and the urinary elimination of uric acid but increased oxypurines and orotidine plasma concentration while urinary excretion of benzbromarone lowers the concentration of uric acid in plasma and increased the excretion of uric acid in urine. However it did not altered the plasma level of uridine or oxypurines or the urinary excretion of oxypurines or orotidine (Tetsuya et al. 1997). Serum uric acid level is typically increased in gout patients. The recommended serum uric acid level must be lowered to a range of < 6 mg/dL for the management of gout symptoms and to decreased acute gout risks (Shoji et al, 2004). Uric acid is excreted through kidney however if kidney function is impaired hyperuricemia may occurred this can also happens in the individuals with normal renal function. If there is a hyperuricemia it may be correlated with incidence of renal impairment and raised the healthcare operation and expenses (Avram and Krishnan, 2008). In case of chronic kidney disease treatment of gout is complicated due to the full treatment by allopurinol. The renal impaired patients had recommended that they should take reduced amounts of allopurinol as they may be at risk for allopurinol toxicity (Hande et al, 1984). Allopurinol is a uric acid lowering drug used in the treatment of gout and the prevention of tumor lysis syndrome. Therapeutic drug monitoring is an important option for evaluation and optimization of allopurinol treatment in case of renal impairment interaction with uricosauric drugs or to verify patient adherence (Mattheus et al. 2007).
Material and Methods. This study was conducted to analyze the urinary excretion and renal clearance of allopurinol and endogenous creatinine in blood and urine. Samples of male gout patients after the oral administration of 300 mg allopurinol were collected. The experiments were conducted on 10 male gout patients. The gout patients who offered to participate was included in this study. The complete demographic data including the age body weight' height, blood pressure and body temperature of gout patients were recoded and presented in Tablet 3.1. Blank blood and urine samples were taken from each gout patients.
2. Sampling Procedure a) Collection of blood samples
The blood samples of each gout patients were collected after 1 and 3 hours of post medication. After the oral intake of allopurinol 300mg (Zyloric) then take serum from these blood samples and stored in ependorf tubes at -20?C until use for the analysis.
3. b) Collection of urine samples
Urine samples of gout patients were collected after 2, 4, 6, 8, 12 and 24 hours after drug administration. These urine samples were stored in plastic bottles in freezer at -20?C until analysis.
4. III.
5. Hplc Analysis
Concentration of allopurinol was determined by HPLC.
IV.
6. Chromatographic System
Chromatography was performed with a high performance liquid chromatography. The HPLC system was consisted of Shimadzu SCL-10A system controller, UV visible SPD-10AV detector and LC-10AT pump with FUC-10AL VP flow controller wall. Separation was achieved at ambient temperature with Hypersil C18 BDS 250x4.6 column pore size of 5 micron. Chromatographic data was collected and analyzed using CSW32 software.
V.
7. Chromatographic Conditions
Quantitative analysis of allopurinol was achieved by using an isocratic mode. UV detector was use for the detection of allopurinol. Hypercil C18 BDS 250*4.6 column was used. Flow rate was maintain at 1ml/min with 20 min run time.
8. a) Preparation of mobile phase
The mobile phase was prepared by dissolving 2.72 g NaCH3COO?3H2O in 3000mL distilled water and correcting the pH to 4.5 with acetic acid 30%. The mobile phase was filtered and degassed before use. The mobile phase was filtered in vacuum filtration assembly having cellulose filter which have pore size 0. 45um (Sartorius company ). Filterd mobile phase was solicited for the removal of bubbles for 10 minutes. (eyela sonicator ) VI.
9. Standards Preparation
Stock solution Stock standard solution of allopurinol (0.25 mg/mL) was prepared in deionized water. Stock solution was stored for further analysis.
10. a) Preparation of working standards in serum
Drug free serum was taken firstly and added working standard of allopurinol of specific concentration 10, 20, 30, 40, 50 and 300?g/ml and mix it with 10% per chloric acid solution (50?L). After cooling in the refrigerator for 10 min dichloromethane (200?L) was added. After shaking the mixture for 30 s, it was centrifuged at 4000 rpm for 5 min. The aqueous supernatant solution was taken. Supernatant was filtered with micro syringe filtration assembly and injected (20?l) into the HPLC instrument for the standard curve. Peak area (mv) versus serum concentration ?g/mL of standard allopurinol was plotted and a linear relationship was obtained. This is given in Table 3.2. Preparation of working standards in urine. Drug free urine was taken firstly and added working standard of allopurinol of specific concentration 10, 20, 30, 40, 50 and 300?g/ml and mix it with 10% per chloric acid solution (50?L). After cooling in the refrigerator for 10 min dichloromethane (200?L) was added. After shaking the mixture for 30 s, it was centrifuged at 4000 rpm for 5 min. The aqueous supernatant solution was taken. Filter and a linear relationship was obtained. The data related to standard concentration of allopurinol in urine were presented in Table this supernatant with micro syringe filtration assembly then injected (20?l) into the HPLC instrument for the standard curve. Peak area (mv) versus urine concentration ?g/mL of standard allopurinol was plotted
11. Calculations a) Diuresis
The rate of urine flow in a time period was calculated as Volume of urine in a collection time period Diuresis mL/min/kg = Volume of urine in a collection time period ______________________________________ Time (min) x body weight (kg)
VIII.
12. Renal Clearance
Renal clearance was calculated by the following formula.
Renal
13. Urinary Excretion
Amount of Allopurinol (mg) excreted in urine at different time intervals were calculated by using formula: Amount excreted (mg) = Concentration of drug (?g/mL) x urine volume _____________________________ 1000
Percentage dose of allopurinol excreted in urine at different time intervals was calculated by formula: Percentage dose (%) = Amount excreted (mg) ___________________ x 100 Amount of dose (mg) Cumulative percentage dose excreted = Cumulative amount excreted __________________________ x 100 Amount of dose (mg)
14. XI. Determination of Allopurinol in Samples
The procedure for determination of allopurinol in serum and urine sample is similar to that of standard solution.
15. Creatinine Analysis
Creatinine analysis was performed by creatinine colorimetric detection kit of (merck company). Some important parameters due to which this method was preferred upon conventional method. The Creatinine colorimetric detection kit utilizes a single-step liquid detection reagent that is safer and less time consuming than other assay methods. This kit is calibrated against the NIST standard and offers reproducible results with less than 6% inter-and intra-assay variation.
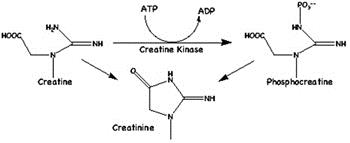
16. Creatinine
(2-amino-1-methyl-5H-imadazol-4one) is a metabolite of phosphocreatine (p-creatine), a molecule used as a store for high-energy phosphate that can be utilized by tissues for the production of ATP. Creatine either comes from the diet or is synthesized from the amino acids arginine, glycine, and methionine. This occurs in the kidneys and liver, although other organ systems may be involved and species-specific differences may exist. Creatine and p-creatine are converted non-enzymatically to the metabolite creatinine, which diffuses into the blood and is excreted by the kidneys. In vivo, this conversion appears to be irreversible and in vitro it is favored by higher temperatures and lower pH. Creatinine forms spontaneously from p-creatine, and under normal conditions, its formation occurs at a relatively constant rate. Intra-individual variation of creatinine levels is <15% from day to day, making it a useful marker for normalizing levels of other molecules found in urine. Altered creatinine levels may be associated with conditions that result in decreased blood flow, such as diabetes and cardiovascular disease.
17. XIII.
18. Statical Calculations
The data on renal clearance was tabulated. The statical calculations were done according to the standard method and results are given as average standard error. The correlation between diuresis and serum concentration of drug and renal clearance was determined by mean off regression / correlation analysis (Steel and Torrie, 2001)
19. Results
The urinary excretion and renal clearance of allopurinol was investigated in ten male gout patients after the oral administration of 300 mg tablet. Blood and urine samples were taken at different time intervals post medication and concentration of allopurinol in each sample was determined by HPLC method. The volume of urine, concentration of creatinine and allopurinol in the urine and serum were measured to calculate diuresis, renal clearance of creatinine and drug and clearance ratio. The results of urinary excretion of allopurinol were expressed in terms of amount excreted in mg, percentage dose excreted, cumulative amount excreted and cumulative percentage dose excreted. Concentration (?g/mL) of allopurinol in urine At 2 hours after drug administration Mean ± SEM value of concentration (?g/mL) of allopurinol in urine was 3.818 ±0.326. At 4 hours after drug administration Mean ± SEM value of concentration (?g/mL) of allopurinol in urine was 23.4160± 5.838. At 6 hours after drug administration Mean ± SEM value of concentration (?g/mL) of allopurinol in urine was 17.30 ± 3.61. At 8 hours after drug administration Mean ± SEM value of concentration (?g/mL) of allopurinol in urine was 11.72 ± 3.18. At 12 hours after drug administration Mean ± SEM value of concentration (?g/mL) of allopurinol in urine was 5.77 ± 1.20. At 24 hours after drug administration Mean ± SEM value of concentration (?g/mL) of allopurinol in urine was 4.16 ± 0.60. Peak concentration in urine of allopurinol is achieved within 6 to 8 hours while before and after this the concentration is very low this is due to the reason that the metabolism of allopurinol inside the human body is slow may be due
20. Figure :
Cumulative amount of allopurinol excreted in urine of ten male gout patients at different time intervals after oral administration of (300 mg) allopurinol.
21. c) Creatinine Concentration in Serum
The mean ± SE value of serum concentration of creatinine was 2.91±0.081?g/ml while it varied from 2.40 to 3.30?g/ml.
22. d) Creatinine Concentration in Urine
Creatinine concentration in urine varied from 3.89 to 29.5?g/ml and its mean value is 18.98±3.63?g/ml.
23. e) Renal clearance of creatinine
Mean ±SEM value for renal clearance of endogenous creatinine was 7.16±3.83 ml/min/kg. While it varied from 0.58 to 33.83 ml/min.kg.
24. Volume XIII Issue VI Version I g) Allopurinol concentration in serum
The mean ± SEM value of serum concentration of allopurinol was 55.76±1.91?g/ml while it varied from 46.76 to 64.76?g/ml.
25. f) Allopurinol clearance
Renal clearance of allopurinol was determined in ten human male gout patients after oral administration of 300 mg allopurinol tablet. Results are presented.
26. h) Allopurinol concentration in urine
Allopurinol concentration in urine varied from 7.34 to 19.36 while its mean ±SE value was 11.039± 1.13?g/mL.
27. i) Renal clearance of allopurinol
The mean ±SEM value of renal clearance of allopurinol calculated was 0.037±0.009 ml/min/kg and it ranged from 0.0004 to 0.0012 ml/min/kg.
28. Table :
Mean data of renal clearance of allopurinol and endogenous creatinine in ten healthy male gout patients after oral administration of allopurinol (100mg)
XV.
29. Discussion
Since the mid-1980s the most frequently used technique in the bio analysis of drugs has been highperformance liquid chromatography (HPLC). HPLC usually exhibits its resolving power at ambient or slightly raised temperatures in the liquid phase with the key requirements being that the analyte has some solubility in the liquid mobile phase and some affinity for the solid stationary phase. It is the relative strength of the analytes affinity for each of these phases that gives the technique its separating capability. Another factor in the emergence of HPLC in pharmaceutical applications has been the types of detectors that may be used generically for wide varieties of drugs and which are compatible with HPLC. The most obvious example is the ultra-violet (UV) absorption detector which has found extremely wide use as most drugs have a chromophore which will absorb UV light of the appropriate wavelength. In HPLC separation occurs due to partitioning between a stationary phase contained in a column and a liquid phase which is pumped under pressure through this column (David N. M., 2004). The principle rout of the drug excretion is the urine. Our kidneys produce urine which contains urea, excess salts, drug metabolites and excess water. Kidneys perform two grand functions.
30. Volume XIII Issue VI Version I
First is to get rid of waste materials and second is to control the composition of the body fluids and the body volume. For water and all electrolytes in the body, balance between output (due to excretion or metabolic consumption) and intake (due to ingestion or metabolic production) is maintained largely by kidneys. The kidneys perform their important function by filtering plasma and removing substances from filtrated at variables rates, depending on the needs of the body itself. Ultimately kidneys clear the wasteful materials from the filtrate by excreting them in the urine while returning substances that are needed by the body back to the blood. Kidneys also eliminate most toxic material and other foreign substances that are either produced by the body or ingested, such as pesticides, drugs and food additives. Renal excretion accounts for most drug correlation between the pH and urine concentration. It means pH did not affect the urinary excretion of drug.
The main functions of kidney are urine formation and water conservation and this is the major channel of water excretion as compared to intestine, skin and lungs (Ganong, 2005). Creatinine is an anhydride end product of creatine metabolism in muscle. The total creatinine in muscle is 10 mg only. The clearance of creatinine is only slightly higher than GFR this metabolite is filtered at the glomerulus but neither secreted nor reabsorbed by the tubules so its clearance gives the GFR. The functional unit of the kidney is nephron 1.2 million nephrons make up each human kidney. The glomerulus is a modified capillary network that delivers an ultra-filtrate of plasma to Bowman's capsule, the most proximal portion of the nephron. These glomeruli collectively produce 120 to 180 liters of ultra-filtrate daily. The volume of the urine excreted (averaging 1.5L/ day or 1mL/ min) represent the sum of two large, directionally opposite processes namely, ultrafiltration of 180L/day and reabsorption of more than 99% of this filtrate by transport process in the renal tubules. Renal blood flow accounts for about 20% of resting cardiac output, yet the kidneys comprise only about 1% of total body weight. This disproportionate allocation of cardiac output is required for the process of ultrafiltration. These processes glomerular filtration, tubular reabsorption and active tubular secretion are involved in the secretion of all metabolites through kidneys (Choi et al., 1993). In ten male gout patients the renal clearance of allopurinol was studied and results have been discussed below. The Mean value of allopurinol renal clearance was 359±9.7 mL/min (Dowling et al., 2001) while another study suggests that the value is 310 ml/min. The mean value of renal clearance of our study was found to be 11.83±0.6.58 mL/min/kg. The difference between these values is due to different temperature and environment. Within 24 hours of oral administration, some 50-70% of the dose mean was excreted in the urine as unchanged drug.
Over the dose range of 0.3-30 mg/kg allopurinol, there was no dose-dependent effect on total or renal clearance 10% of a allopurinol dose being excreted unchanged in urine with the major site of elimination which occurs by renal mechanisms. At the glomerulus allopurinol is mainly secreted. The difference in the urinary excretion of allopurinol under local conditions and reported in literature is due to environmental and genetic influences on glomerular filtration rate which significantly affect the fate of drug in the body. These differences have been elucidated by original term geonetics (Jeffrey et al, 1998). Studies on allopurinol suggest that it is extensively secreted from urine even though when given in small amounts. At serum concentrations up to 30-fold the tubular secretion rate of allopurinol gradually increases and higher than those values which are achieved during 300 mg/day typical oral dosing. The retention time for the present study was 9 min for plasma and 10 min for urine. The difference is probably due to storage of urine and plasma samples, environmental conditions and/or temperature. The present percent dose is lower calculated as 66.30±2.18.
There is difference between present study value and earlier study value due to difference in environment, temperature but major difference in the values is due to non-fasting gout patients (Konrad et al. 2002).
drug and its absorption from the tubules. Most of the drugs are either weak acids or weak bases. Acidic drugs are more readily ionized in alkaline urine and alkaline drugs are more readily ionized in acidic urine. Ionized or polar substances are more soluble in water so readily dissolve in the body fluids for excretion (Lin et al., 1988). The present study revealed the result non-significant control the excretion of certain drugs from the body. Urine pH plays an important role in the ionization of the elimination that are predominately ionized at physiological pH and for polar drugs, drug metabolites with low lipid solubility. Renal drug excretion decreases with aging. Drugs bound to plasma proteins remain in the circulation; only unbound drug is contained in the glomerular filtrate. Un-ionized forms of drugs and their metabolites tend to be reabsorbed readily from tubular fluids (Guyton and Hall, 2000).Urine pH has a great influence on whether a drug is excreted readily or slowly and in some clinical situations urine pH is maintained to

| Urinary |
| Sr. No | Concentration (µg/ml) | Peak area(mv) |
| 1 | 10 | 7.5 |
| 2 | 20 | 10.7 |
| 3 | 30 | 13.3 |
| 4 | 40 | 29.1 |
| 5 | 50 | 32.7 |
| 6 | 300 | 153.25 |
| Volume XIII Issue VI Version I |
| XIV. |