1. Introduction
hree dimensional ultrasound examination has been introduced to evaluate fetal blood flow and vascularization in several organs, such as kidneys, liver and brain [1] in normal pregnancies. Moreover this technique has been applied to assess placental circulation.
An estimated fetal weight (EFW) less than the 10th percentile has been most widely applied as the threshold to define FGR, according to ACOG guidelines. [2] Fetal growth restriction is characterized by important haemodinamic changes, with a redistribution of blood flow towards vital organs, such as brain, heart and adrenals despite other districts (abdomen). This blood flow centralization process is defined as "brain sparing effect", traditionally identified by a reduced Doppler pulsatility (PI) in the middle cerebral artery (MCA).
FGR affects 5-10% of pregnancies and represents mainly a complication of placental dysfunction. It is associated with significant perinatal mortality and morbidity and even increased risk for poor long-term outcomes, involving general cognitive competence.
Overt brain lesions such as hypoxic-ischemic encephalopathy, intraventricular hemorrhage and leukomalacia can occur in up to 15% of all FGR fetuses, while a substantial proportion of FGR infants could present subtler neurobehavioral disturbances. 3,4 Chronic hypoxia due to placental insufficiency cause a blood flow centralization process, also known as "brain-sparing effect", which has been considered an adaptative response of the fetus, in order to maximize brain oxygen supply. It has been classically identified by reduced Doppler pulsatility index (PI) in the middle cerebral artery (MCA). [5][6][7][8] Two different populations of fetal growth restricted fetuses have been identified depending upon the gestational age in which FGR occurs. These populations present different patterns of deterioration that can be investigated in multiple vascular beds, using power Doppler ultrasound. Early-onset FGR, presenting before 34 gestational weeks, is first characterized by an escalation in blood flow resistance in umbilical artery (UA), accompanied by vasodilatation of MCA, then followed by deterioration of venous Doppler parameters and biophysical profile score (BPS). In late-onset FGR, beyond 34 gestational weeks, normal or only mildly elevated UA Doppler parameters with an isolated MCA vasodilatation can be found.9
The main purpose of antenatal surveillance remains the identification of the best moment for delivery balancing neonatal and fetal morbidity and mortality.
The aim of this study is to explore the possible use of 3D power Doppler ultrasound angiography (3D-PDA) using VOCAL software (GE Healthcare, USA) in the assessment of different cerebral regions in lateonset growth restricted fetuses versus normal ones.
2. II.
3. Materials and Methods
Between January 2011 and February 2012 a group of 28 consecutive cases of singleton pregnancies affected by late onset (34-36 weeks of gestation) growth restriction and 77 appropriate for gestational age fetuses (AGA) have been enrolled in the study.
FGR is defined as an ultrasound-estimated fetal weight below the 10th percentile for gestational age according to the Hadlock 4 equation, using biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC) and femur length (FL). 10 We enrolled only fetuses with a late-onset growth restriction, that is to say it has occurred after 34 gestational weeks9, and with a maximum gestational age of 36 weeks.
Exclusion criteria were as follows: 1) multiple pregnancy, 2) fetal malformation or chromosomal defects, 3) maternal complications, 4) conception by assisted reproductive techniques.
All ultrasound examinations were performed by a single operator (A.R.) using General Electric E8 (General Electric Corp. Milkwaukee, WI, USA) with a 5MHz trans-abdominal probe equipped with automatic volume measurements, colour, pulsed and power Doppler options.
Before starting the 3D examinations we calculated fetal biometry ( BPD, HC, AC, FL, EFW), amniotic flow volume and maternal uterine arteries Doppler.
Pulsed-wave Doppler flow analysis of the umbilical artery (UA) was obtained from a free-floating central section of the cord with an angle close to 0°, while the middle cerebral artery (MCA) was sampled at the proximal end of the vessel close to the circle of Willis with an insonation angle of about 0°. Three subsequent blood velocity waveforms for each vessel were analyzed for PI according to Gosling et al.11 We checked the results against previously published reference ranges12-13 and defined abnormal Doppler when PI showed at least 20% deviation from the mean value. 14 The introduction of 3D power Doppler (3D-PD) and the vascularization histogram allowed to quantify the vascularization and blood flow to the placenta and several fetal organs.
Moreover, power Doppler does not show aliasing effect and the colour map is independent of insonation angle. 15 The use of 3D-PD is particularly useful in the evaluation of fetal brain vessels because of their small caliber. 3D-PDA images of the fetal brain were acquired during fetal rest, using the same presets for each acquisition. The angle of acquisition was set at 35°, the pulsed repetition frequency (PRF) of the power Doppler at 0.9. We chose the biparietal plane including landmarks like the thalami, the third ventricle, the cavum septi pellucidi (CSP), the tentorial hiatus and a symmetrical display of the calvaria for recording power Doppler signals.
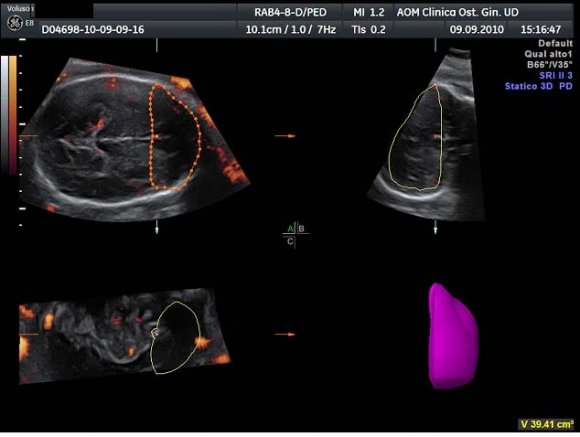
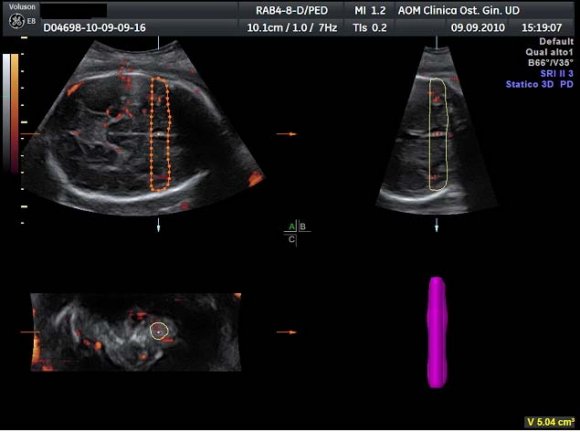
After displaying three simultaneous perpendicular planes on the monitor (axial, sagittal and coronal) the size of the region of interest (ROI) was adapted manually to created the two zones of the fetal brain to be analyzed (Fig. 1). These two ROI were defined by using anatomy landmarks to ensure a good reproducibility of this method among different operators. The first ROI is the Frontal Zone (zone 1), which has been obtained by tracing the contour of the anterior part of the fetal brain up to the perpendicular line crossing the anterior edge of the CSP (Fig. 2). The second ROI, Temporal Zone (zone 2), is defined by a rectangle reaching from both temporal bones with the width of CSP included (Fig. 3).
The volume of the investigated zones and the blood flow indexes were calculated using VOCALTM software. A rotation step for each contour plane was selected with a 30° angle chosen arbitrarily. This procedure of rotating the reference plane was done until a full rotation of 180° was achieved. The fetal brain volumes were calculated after all contour traced (6 steps). Eventually, the Vocal Histogram switch was activated for the automatic calculation of the 3D-PDA vascular indexes. Three vascular indexes were generated: vascularization index (VI) defined as the percentage of power Doppler data (coloured voxels) within the volume of interest; flow index (FI), the mean signal intensity (average colour value) of the power Doppler information; vascularization-flow index (VFI), a combination of both factors derived through their multiplication. 16 Inter-and intraobserver reproducibility was assessed with the intraclass correlation coefficient.
Difference between AGA and growth-restricted fetuses were evaluated using Student's test. P<0.05 was considered significant.
The study was approved by the local Ethics Committee and written consent was obtained from all participants.
Volume XIV Issue I Version I Year ( ) III.
4. Results
A total of 105 pregnant women with a gestational age ranges from 34+0 to 36+0 weeks were included in the present study. The mean maternal age was 30.6±3.1; 43% of the women was primigravida and 57% was multigravida respectively; a total of 46% was primipara whereas 54% was multipara.
All the fetuses included in the late-onset FGR group (28 fetuses) had normal UA PI and normal MCA PI.
In the table 1 are reported the values of the vascular parameters (VI, FI, VFI) in Frontal Zone (zone 1) for the FGR group and the control group. VI and VFI were both increased in the FGR group with statistical significance comparing to control group (P<0.05).
Table 2 shows the values of VI, FI, VFI in Temporal Zone (zone 2) for FGR group and control group. VI and VFI were significantly decreased in the FGR group comparing to the control group.
IV.
5. Discussion
In our study all the fetuses with late-onset FGR demonstrated no alterations in bidimensional Doppler. In late-onset FGR cardiovascular abnormalities are typically more subtle and do not extend beyond the cerebral circulation.9,17 Almost all clinical studies have focused on the assement of MCA Doppler, which is still considered as the clinical standard for the hemodynamic evaluation of the fetal brain.8 Indeed, the "brainsparing" onset has been classically identified by a reduction in MCA PI.5-7
However, recent longitudinal studies based on power Doppler evaluation of different brain arteries in growth restricted fetuses emphasize that MCA PI is reduced in a later stage than the anterior cerebral artery (ACA). It probably means that brain blood perfusion in FGR follows an internal regional redistribution, which changes with the progression of hypoxic fetal deterioration.3,18-20
Our findings agree with these observations. The VI and VFI we obtained by 3D-PDA seem to demonstrate regional changes in blood perfusion, which appears increased in the Frontal Zone (zone 1) and decreased in the Temporal Zone (zone 2) respectively, compared with the control group. The VI has been suggested to be representative of the number of vessels in the ROI21, but recently Jones et al. 16 specified that an increased VI in the ROI can be due both to an increased dimension of a vessel (vasodilatation) and to diversion to other vessels secondary to pressure rise, showing a strong linear relationship to volume flow rates. 22 In contrast, FI is less predictable and seems to have a more complex, non-linear relationship to flow rates.17 VFI obviously feels the effects of both previous indexes.
The initial preferential increment in blood supply to the Frontal Zone can be associated with preservation of general cognitive functions such as impulse control, language, memory, problem solving and suggests a hierarchical order in the protection of brain functions.23 Moreover, three dimensional indices were easy to obtain and showed a high level of intra-and interobserver repeatability as reported in previous papers (24) .
With this in view, MCA vasodilatation (MCA PI reduction) may do not represent a protective response but rather the starting point after which the protection of the frontal area begins to decline. The real "brainsparing effect" seems to be marked by hemodynamic changes in the anterior cerebral artery (ACA) and consequently in its districts. If confirmed, these findings might have important implications, especially since Doppler findings may be subtle and accurate identification of growth restriction arising in the third trimester still provides a challenge. The clinical significance of the observations reported in the present study remains to be established by larger prospective studies with long term postnatal neurological follow-up.
Finally, according to the results we obtained in this study, 3D sonography and power Doppler angiography can be considered as new techniques offering additional vascular parameters allowing for detection of early non invasive "brain sparing markers" in fetuses affected by FGR, even without any pathological 2D Doppler velocimetry. Construction of reference charts and interobserver variability study of 3D-PDA vascular indexes of fetal brain circulation in normal pregnancies need to be planned.
V.
6. Aknowledgments
The Authors report no conflicts of interest.

| TEMPORAL ZONE | VI | FI | VFI |
| (ZONE 2) | |||
| Late-onset FGR | 0,9* (0,3) | 29,5 | 0,2* (0,1) |
| (28 fetuses) | (7,5) | ||
| Control Group | 3,4 (0,7) | 27,7 | 1,2 (0,4) |
| (77 fetuses) | (6,0) | ||
| * P<0,05 vs Controls (Student's t-test) and p<0,05 vs Group 1(ANOVA) | |||
| FIGURES SECTION | |||