1. Introduction
ncreased mucus secretion is a clinical feature of severe respiratory diseases, such as asthma, cystic fibrosis and chronic obstructive pulmonary disease [1]. Pharmacological approaches for relieving mucus hypersecretion currently include several classes of agents, including expectorants, mucoregulators, mucolytics, bronchodilators anti-inflammatory drugs and antioxidants [2]. Classic mucolytic drugs such as Nacetylcysteine decrease the viscoelastic properties of mucus by reducing disulfide bonds. In contrast, expectorants change mucus consistency and make coughing more productive, mucokinetics improve transportability, and mucoregulators suppress mucus secretion. Mucolytics generally decrease mucus viscosity by reducing the dicysteine bridges that contribute to the rigidity of the mucins [3]. Guaifenesin (GFN) is a commonly used expectorant drug for productive cough, which is reported to increase the volume and reduce the viscosity of tenacious sputum [4,5].
Currently recommend consideration for management of mucus hypersecretion is the combination of expectorants, mucoregulators, mucolytics and even bronchodilators in different multi drug components pharmaceutical formulations [3,6]. Therefore, the simultaneous identification and quantification of active pharmaceutical ingredients (API) and its related impurities along with some other active ingredients and excipients in multicomponent pharmaceutical products is a very intensive activity performed at many levels of the drug discovery pipeline and beyond. Impurities relate to starting materials, byproducts, breakdown products or polymorphs are of significant concern as they may carry activity responsible for eventual undesirable side effects or toxicity and may interfere with the drug's activity. Thus monitoring impurities in API which exist as various combinations in cough-cold multicomponent drug products is a prerequisite for insuring drug safety and quality.
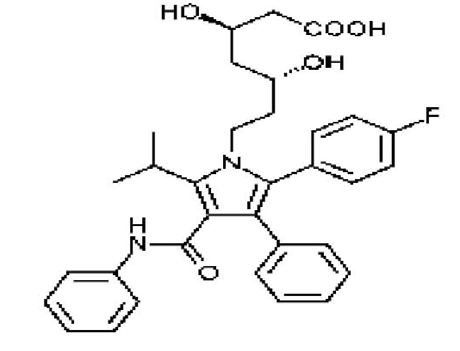
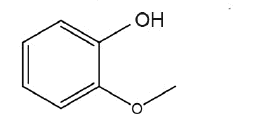
A literature survey reveals some HPLC methods that are reported for the simultaneous determination of GFN along with some other active ingredients in a multicomponent tablet and liquid dosage formulation as anticipated with the variation of mobile phase, column and detector. Different HPLC methods for individual assay and related impurities are available for GFN in official pharmacopoeia and several LC-MS/MS methods were used for determination of GFN in Human Plasma [11]. Hence an attempt has been made to develop a simple, efficient and selective method for the determination of guaifenesin impurities (Figure 1), 2-(2methoxyphenoxy)-propane-1,3-diol ( -isomer) and 2-methoxyphenol (guaiacol) in the presence of guaifenesin, ambroxol hydrochloride and salbutamol sulfate in multi drug components pharmaceutical formulations.
2. II.
3. Materials and Methods
4. a) Instrumentation
A High Performance Liquid Chromatography (HPLC) method for GFN -isomer and guaiacol analytical method was developed on PLATIN BLUE UPLC (Knauer, Germany) with diode array detector. NUCLEODUR-100-3C18 (250x4, 6 mm, 3 m packing, Machery-Nagel, Germany) column was used. The elution was carried by gradient elution method of mobile phases A and B.
5. b) Chemicals
Ammonium acetate (Sigma-Aldrich, HPLC grade), Millipore water, methanol (HPLC grade, Alpha chemika, purity: 99.9%, batch: A----); acetornitrile (HPLC grade, Alpha chemika; purity: 99.9%, batch: A5982;), impurity A, 2-methoxyphenol (guaiacol) Sigma-Aldrich, purity: 99,9%); impurity B 2-(2-methoxyphenoxy)propane-1,3-diol ( -isomer) (Sigma-Aldrich, purity: 99,9%); GFN and ambroxol hydrochloride (Sigma-Aldrich, purity: 99,9%); salbutamol sulfate (Sigma-Aldrich, purity: 99,8%); methylparaben (Sigma-Aldrich, purity: 100%); propylparaben (Sigma-Aldrich, purity: 100%) and citric acid monohydrate (Sigma-Aldrich, purity: 99.7%) were used in this study.
6. c) Preparation of stock solution and working standard solution
Preparation of mobile phase. Solvent A -7.7 gm of ammonium acetate was weighed and transferred into a 1000 ml beaker, dissolved and diluted with 1000 ml water and pH brought to 6.8 by ammonia or acetic acid. The solvent A was filtered through 0.45 m membrane filter under vacuum filtration and was degassed before used, then delivered at a flow rate 1.0 ml/min. Solvent B -acetonitrile and methanol (80:20). d) Preparation of solvent for standards and sample Solvent C-750 ml of solvent A and 250 ml of solvent B are mixed together. e) Preparation of standard solution 10.0 mg of GFN standard was weighed and transferred into 10 ml volumetric flask. 8 ml of solvent C was added sonicated for 5 min, mixed thoroughly to dissolve and make up the volume to 10 ml with mobile phase (1 mg/ml concentration). 5.0 mg of guaiacol reference standard was weighed and transferred into 20 ml volumetric flask and make up the volume to 20 ml with solvent C. 1.0 ml of guaiacol solution was transferred into 50 ml volumetric flask and make up the final volume to 50 ml with solvent C (5 g /ml concentration). 10.0 mg of GFN -isomer reference standard was weighed and transferred into 20 ml volumetric flask and make up the volume to 20 ml with solvent C. 1.0 ml of GFN -isomer solution was transferred into 50 ml volumetric flask and make up the final volume to 50 ml with solvent C (10 g/ml concentration).
7. f) Preparation of sample solution
Melon® (Aversi, Georgia) which is a combination of ambroxol hydrochloride (15 mg); salbutamol sulfate (2.4 mg); guaiphenesin (100 mg), per 190 mg tablet or a cough mixture of ambroxol hydrochloride (15 mg); salbutamol sulfate (1.2 mg); guaiphenesin (50 mg), per 5 mL syrup were used in this study. 20 tablets were grinded in to a homogenous powder and 190 mg were transferred into 100 ml volumetric flask and make up the final volume to 100 ml with solvent C (1mg/ml concentration). 10.0 ml of the syrup was transferred into 100 ml volumetric flask and make up the final volume to 100ml with solvent C (1mg/ml concentration). The sample solutions were sonicated for 5 min, mixed thoroughly to dissolve and filtered through 0.45 m membrane filter.
8. g) Specificity and Robustness
The specificity of the assay method is established by injecting blank, containing 1 mg/ml GFN, ambroxol hydrochloride, salbutamol sulfate methyl-, propylparaben and citric acid monohydrate as well as standard and samples into the HPLC. The identity of GFN impurities, including -isomer and guaiacol was confirmed by comparison of its retention time (RT) and UV-spectra. Robustness was established by varying the chromatographic condition with respect to specificity of the method in various pH conditions of mobile phase. Standard and sample solutions were injected and the chromatograms were recorded. h) Quantification Limits
The quantification limit was defined as the lowest fortification level evaluated at which acceptable average recoveries were achieved and analyte peak is Where 'S' is the standard deviation of replicate determination values; 'K' is the sensitivity namely the slope of the calibration graph.
9. i) Calibration curve
The calibration curve was constructed by plotting peak area concentration of GFN impurities standard solutions. Aliquots of guaiacol standard stock solutions in the concentration range 0.1-10 g/ml and GFN -isomer reference standard in the concentration range 1.0 -100 g/ml were transferred into 25 ml volumetric flask and 10 ml of solvent C was added, sonicated for 5 min, mixed thoroughly to dissolve and make up the volume to 25 ml with solvent C. Each concentration of the standard solutions 10 l was injected and the chromatograms were recorded. The calibration graph was done by external standard calibration and confirmed using back calculation method.
10. j) Accuracy
Accuracy was determined for standard quality samples (in addition to calibration standard) prepared in triplicates at different concentration levels (5.0, 50, 100 µg/ml for GFN -isomer and 0.5, 5.0, 10.0 µg/ml for guaiacol standard solutions respectively.) within the range of linearity of GFN impurities. The results of analysis of recovery studies were obtained by method validation by statistical evaluation.
11. k) Precision
The precision of the instruments was checked by repeatedly (intra day) intermediate (inter day) and reported as % RSD for a statistically significant number of replicate measurements. Repeatability and intermediate precision of the method were determined by analyzing 6 samples of the test concentration 5.0, 50, 100 µg/ml for GFN -isomer and 0.5, 5.0, 10.0 µg/ml for guaiacol standard solutions respectively.
12. l) Stress Conditions
The stress conditions employed for degradation study included oxidative hydrolysis and photochemical degradation as it described in [12]. To 10 ml of both GFN standard solution and pharmaceutical formulations 10 ml of 1 % v/v H2O2 was added separately. These mixtures were refluxed separately for 1 hour at room temperature. The forced degradation in oxidative media was performed in the dark in order to exclude possible photo-degradation. For carrying out photolysis studies the samples were treated with UV light for 6 hours at 254 nm and also in sunlight.
13. III.
14. Results and Discussion
15. a) Method development
The aim of this study was to develop a simple, efficient and selective method for the determination of GFN impurities 2-(2-methoxyphenoxy)-propane-1,3-diol ( -isomer) and 2-methoxyphenol (guaiacol) in the presence of GFN, ambroxol hydrochloride and salbutamol sulfate in multi drug components pharmaceutical tablet and syrup formulations. Various attempts were made to separate all degradation products with different pH of the mobile phase buffer and composition of methanol in the mobile phase using C-18 and C-8 stationary phase columns. The RP-HPLC method for GFN -isomer and guaiacol was optimized (Table 1). To ensure great resolution between all known and unknown degradation compounds, the C-18 stationary phase with an end-capping was used. HPLC parameters, such as detection wavelength, ideal mobile phase & their proportions and flow rate were carefully studied (Table 1). After trying different ratios of mixtures of methanol:acetonitrile and ammonium acetate buffer the best results were achieved by using a gradient elution. The mobile phase gradient constituted by ammonium acetate buffer: (solvent A) and acetonitrile: methanol (80:20) (solvent B). At a flow rate of 1.0 ml/min, the retention time were 6, 32 min for guaiacol and 12, 73 min for GFN -isomer. The analytes peak areas were well defined and free from tailing under the described experimental conditions. b) System suitability System suitability test was carried out on freshly prepared solution of GFN -isomer and guaiacol to ensure the validity of the analytical procedure. Data from six injections were used to confirm system suitability parameters like retention time, UV-spectra and peak area. The results are presented in Table 2. The values obtained demonstrated the suitability of the system for the analysis of GFN impurities. The method gives sharp and well defined peaks with significant RT values which were desired for quantification of GFN related impurities in the presence of blank, containing GFN, ambroxol hydrochloride and salbutamol sulfate (Table 2).
16. c) Specificity
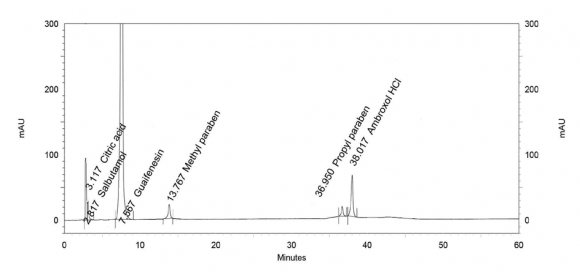
Specificity is the ability of the method to measure the analytes response in the presence of their potential impurities and degradation products. Blank (placebo) interference was evaluated by analyzing the blank, containing GFN, ambroxol hydrochloride, salbutamol sulfate methyl-, propylparaben and citric acid, prepared as in the test method (Figure 2a). The method showed specificity because GFN -isomer and guaiacol were well-resolved and no interfering peaks were observed as it appears in Figure 2b. Stress studies were performed either for guaifenesin impurities and tablet to provide an indication of the stability-indicating property and specificity of proposed method. The stress conditions employed for degradation study included oxidative hydrolysis and photochemical degradation. GFN -isomer and guaiacol were found stable under oxidative and photolytic stress conditions (Figure 3). The peak purity test was carried out for the guaifenesin peak by using the PDA detector in stress samples. The mass balance (% assay + % sum of all degradants + % sum of all impurities) results were calculated and found to be more than 95%. The purity of GFN -isomer and guaiacol was unaffected by the presence of GFN, ambroxol hydrochloride, salbutamol sulfate methyl-, propylparaben and citric acid and degradation products, and thus confirms the stability-indicating power of the developed method.
17. d) Linearity and LOQ
The linearity was determined by constructing calibration curve. The calibration curves in this study were plotted between amount of each of analyte versus peak area and the regression equations with a regression coefficient were obtained. The linear regression data (Table 3) showed good linear relationship over a concentration range of 1-100 µg/ml for GFN -isomer and 0.1-10.0 µg/ml for guaiacol. Regression equation for GFN -isomer was Y=7.709X + 0.165 and Y=5.588X + 0.005 for guaiacol with a regression coefficient of 0.9999 for each of analyte. The linearity of estimated RP-HPLC method was found to be over the concentration range of 1-100 µg/ml for GFNisomer and 0.1-10.0 µg/ml for guaiacol which furthermore have been confirmed using back calculation method. The RE % of linearity back calculation method requirements for analyte calculated to introduced concentration ration to be less than 15% for at last 6 calibration standards or 75 % of samples, expect LOQ, which should be not less than 20%. As it shown in the Table 3, the GFN -isomer and guaiacol RP-HPLC assay linearity meets all the validation quality requirements.
18. e) Accuracy and precision
The intra day precision was determined by measurement of analyte concentration using five replicates of GFN impurities solutions at three different concentrations 0.5; 5 and 10 g/ml for guaiacol and 5,0; 50 and 100 g/ml for GFN -isomer two times on the same day and inter day variations were determined similarly on consecutive days. These concentrations have been selected according to the assay quantification low, medium and high limits for each of analyte (QCL, QCM and QCH respectively). The repeatability of sample application was assessed 5 times on HPLC followed by recording of the amount of GFN related impurities solutions. The % RSD for peak values of guaiacol was found to be 2.188% and 2.591% for QCL intra and inter-day precision respectively. The % RSD and results for GFN related impurities QCL, QCM and QCH concentration are depicted in Table 4, which reveal intra and inter day variations of analytes concentration.
19. f) Recovery studies
The accuracy of the proposed method was also further assessed by performing recovery experiments using the standard addition method. Recovery studies of the different samples were carried out for the accuracy parameter. These studies were carried out at three levels (QCL, QCM and QCH respectively); sample solutions of 5, 50 and 100 g/ml as well as standard solutions were prepared for the GFN -isomer and recovery studies were performed using five replicates. For guaiacol accuracy parameter studies three concentration levels either of sample solutions as well as standard solutions QCL, QCM and QCH, corresponding to 0.5, 5.0 and 10.0 g/ml concentration respectively were used. The repeatability of sample application was assessed 5 times on HPLC followed by recording of the peak area of GFN related impurities solutions. Percentage recovery was found to be within the limits as listed in Table 5.
20. g) Robustness
To determine the robustness of the developed method, experimental conditions were deliberately altered and the relative retention time of -isomer and guaiacol with respect to guaifenesin; and system suitability parameters for guaifenesin standard was recorded. The variables evaluated in the study were pH of the mobile phase buffer (±0.2), column temperature (± 5°C). In all the deliberate varied chromatographic conditions, all analytes were adequately resolved and the elution order remained unchanged.
21. IV.
22. Conclusion
A new, accurate and selective HPLC method were proposed for the determination of guaifenesin impurities, 2-(2-methoxyphenoxy)-propane-1,3-diol (isomer) and 2-methoxyphenol (guaiacol) in the presence of guaifenesin, ambroxol hydrochloride , salbutamol sulfate in multi drug components pharmaceutical formulations as per the ICH guidelines. The methods were found to be simple, selective, precise and accurate. Therefore, these methods can be used as routine testing as well as stability analysis of guaifenesin and ambroxol impurities in bulk and in formulations.
23. B
In order to determine the quantification limit analytes concentration in the lower part of calibration curve was used. GFN -isomer and guaiacol solutions of 1 g/ml and 0.1 µg/ml respectively were prepared and analyzed using six replicates and the amount of each analyte peak area was determined. The LOQ values for GFN -isomer and guaiacol are shown in Table 3.
difference and recovery %) were within the acceptance criteria.
V.

| ambroxol impurities | |||||
| Parameter/Condition | Specification | ||||
| Column | |||||
| Mobile phase gradient | Solvent A-0.1 M ammonium acetate buffer of pH 6.8 Solvent B -acetonitrile:methanol (80:20) | ||||
| Working wavelength | 275 nm | ||||
| Column temperature | 45 o C | ||||
| Sample volume | 10 uL | ||||
| Run time | 60 min | ||||
| Time (min) | Flow | Comp. A | Comp. B (%) | ||
| (ml/min) | (%) | ||||
| 1 | 0.0 | 1.0 | 25 | 75 | |
| . | |||||
| 2 | |||||
| . | |||||
| Gradient elution | |||||
| Parameters | GFN ?-isomer | |
| R T | 12,726 ± 0,0087 | 6,318 ± 0,060 |
| Peak area | 17517,4 ± 417,17 | 11591.6 ± 180,76 |
| R T %RSD ¶ | 0,068 | 0,95 |
| Peak area %RSD ¶ | 2,381 | 1,559 |
| method | ||
| Parameters | guaiacol | GFN ?-isomer |
| Concentration range | 0.1-10 µg/ml | 1-100 µg/ml |
| Slope | 5.588 | 7.709 |
| Intercept | 0.005 | 0.165 |
| Correlation coefficient | 0.9999 | 0.9999 |
| Regression equation | Y=5.588X + 0.005 | Y=7.709X + 0.165 |
| RE%* | -2.051 | 0.175 |
| LOQ (µg/ml) | 0.098 ± 0.0029 | 1.017 ± 0.0109 |
| LOQ %RSD | 2.974 | 1.078 |
| Parameters |
| Year 2014 | ||||||
| 32 | ||||||
| Volume XIV Issue II Version I | ||||||
| ( ) B | ||||||
| Medical Research | ||||||
| Global Journal of | GFN ?-isomer | |||||
| Parameters | QCL | QCM | QCH | QCL | QCM | QCH |
| 0.5 ?g/ml | 5 ?g/ml | 10 ?g/ml | 5 ?g/ml | 50 ?g/ml | 100 ?g/ml | |
| Peak area of | 8697 ± | 87717± | 180311 ± | 60141 ± | 624380 ± | 1369669 ± |
| sample* | 249.2 | 832.2 | 2290 | 780.7 | 18210 | 44541 |