1. Introduction
ntioxidant can be defined as substances that inhibit the oxidation of the other molecules. Reactive oxygen species (ROS), naturally generated during the metabolism, can damage biological structures such as proteins, lipids or DNA. Normally, cells defend themselves against ROS damage with enzymes, but sometimes the natural defenses are overwhelmed by an excessive generation of ROS and a situation of oxidative stress occurs. In this case, cellular and extracellular macromolecules (proteins, lipids, and nucleic acids) can suffer oxidative damage, causing tissue injury [1,2].
Antioxidant compounds in food are found to have a health protecting factor. Primary sources of naturally occurring antioxidants are whole grains, fruits and vegetables.
Garlic (Allium sativum) is an herb. It is best known as a flavoring for food [15]. But over the years, garlic has been used as a medicine to prevent or treat a wide range of diseases and conditions. The fresh clove or supplements made from the clove are used for medicine [16]. Garlic is used for many conditions related to the heart and blood system. These conditions include high blood pressure, high cholesterol, and coronary heart disease, heart attack, and "hardening of the arteries" (atherosclerosis) [17]. Some of these uses are supported by science. Garlic actually may be effective in slowing the development of atherosclerosis and seems to be able to modestly reduce blood pressure. Some people use garlic to prevent colon cancer, rectal cancer, stomach cancer, breast cancer, Author ? ? ?: Equipe d' Electrochimie Moléculaire et Matériaux Inorganiques, Université Sultan Moulay Slimane, Faculté des Sciences et Techniques de Beni Mellal, Maroc. e-mail: [email protected] prostate cancer, and lung cancer. It is also used to treat prostate cancer and bladder cancer. Garlic has been tried for treating an enlarged prostate (benign prostatic hyperplasia; BPH), diabetes, osteoarthritis, hayfever (allergic rhinitis), traveler's diarrhea, high blood pressure late in pregnancy (pre-eclampsia), cold and flu. It is also used for building the immune system, preventing tick bites, and preventing and treating bacterial and fungal infections. Other uses include treatment of fever, coughs, headache, stomach ache, sinus congestion, gout, rheumatism, hemorrhoids, asthma, bronchitis, shortness of breath, low blood pressure, low blood sugar, high blood sugar, and snakebites. It is also used for fighting stress and fatigue, and maintaining healthy liver function. Some people apply garlic oil to their skin to treat fungal infections, warts, and corns. There is some evidence supporting the topical use of garlic for fungal infections like ringworm, jock itch, and athlete's foot; but the effectiveness of garlic against warts and corns is still uncertain. There is a lot of variation among garlic products sold for medicinal purposes. The amount of allicin, the active ingredient and the source of garlic's distinctive odor, depends on the method of preparation. Allicin is unstable, and changes into a different chemical rather quickly. Some manufacturers take advantage of this by aging garlic to make it odorless. Unfortunately, this also reduces the amount of allicin and compromises the effectiveness of the product. Some odorless garlic preparations and products may contain very little, if any, allicin [18,19].
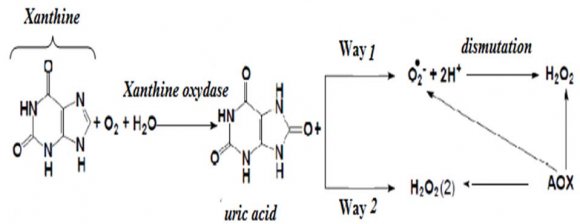
Several amperometric biosensors have already been proposed for antioxidant capacity evaluation [3][4][5][6][7][8][9][10][11][12][13][14]. Most of them are based on the amperometric detection of H 2 O 2 , resulting from the catalyzed dismutation of superoxide radicals (O 2 -.) in presence of superoxide dismutase.
In this work, electrochemical deposition of copper on paste carbon electrode is carried out to develop stable recognition layers for the voltammetric detection of antioxidant capacity of domestic garlic, tea and coffee samples.
The antioxidant capacity was evaluated, by coupling an amperometric sensor for H 2 O 2 detection, obtained by modification of paste carbon graphite electrode with copper, with xanthine oxidase (XOD) immobilized at silice -xanthine (XA) enzymatic system, as generator of O 2 -. radicals. The advantages of this strategy consist to:
? It works at low applied potential, allowing a significant decrease of the risk of electrochemical interferences;
? The antioxidant capacity evaluation, requiring the monitoring of H 2 O 2 concentration in presence of antioxidant sample as well as in its absence, will enhance global estimation of free radicals (O 2 -.) or no radical reactive species, (H 2 O 2 ) (Reaction 1).
2. Reaction 1
II.
3. Experimental Section a) Apparatus
Electrochemical experiments were performed using a voltalab potentiostat (model PGSTAT 100, Eco Chemie B. V., Utrecht, The Netherlands) driven by the general purpose electrochemical systems data processing software (voltalab master 4 software).
All the electrochemical experiments were performed in a standard one-compartment threeelectrode cell. The reference electrode was SCE and the counter electrode was platinum. All electrode potentials were referred to this reference electrode. The working electrode was copper modified carbon paste electrode (Cu-CPE).
4. b) Reagents and Solutions
All chemicals were of the highest quality. Graphite powder (spectroscopic grade RWB, Ringsdorff-Werke GmbH, Bonn-Bad Godesberg, Germany) was obtained from Aldrich and was used without further purification. CuSO4 was obtained from Merck chemicals. Deionised water was used to prepare all solution.
5. c) Preparation of the Electrochemical Sensor
The carbon paste unmodified was prepared by adding paraffin oil to carbon powder and thoroughly hand -mixing in a mortar and pestle. The resulting paste was packed into the electrode and the surface was smoothed. The electrochemical sensor was developed by depositing the copper at fixed potential (0.1 V for 1 hour) onto the carbon paste electrode surface.
6. d) Procedure
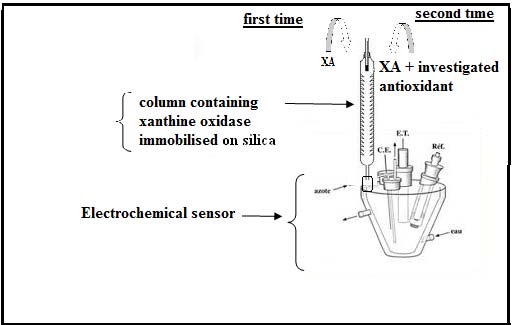
The device constructed for the measurement of the antioxidant capacity is given in Figure 1. The free radical was generated in column following the reaction 1, a calibration curve; giving current density of H 2 O 2 reduction versus [H 2 O 2 ] is recorded. In the second test, the investigated antioxidant associated to xanthine solution, were pouring in column and electrochemical response behaviour was recorded, the [H 2 O 2 ] no consumed was evaluated from calibration curve already established.
7. Results and Discussion
8. a) Characterization of Prepared Electrode
The cyclic voltammograms (CVs) of the copper modified carbon paste electrode (Cu-CPE) and carbon paste electrode (CPE) were recorded in the supporting electrolyte (phosphate buffer solution) (Fig. 2).
We can see that the shape of the cyclic voltammogram was modified in the presence of copper at CPE surface, suggesting that the carbon paste electrode was effectively modified by copper. The surface structure of copper modified carbon paste surface was observed using scanning electron microscopy (Fig. 3). The film layer of copper was formed on the surface of carbon paste electrode; it was not disintegrated or detached from the surface when immersed in the buffer solution.
9. b) Calibration Graph
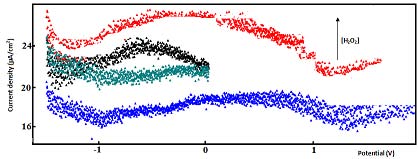
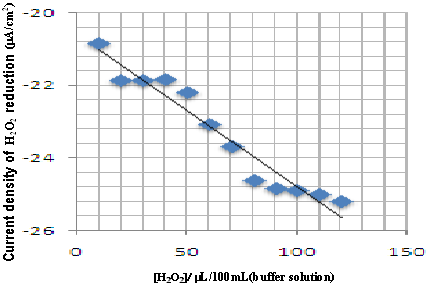
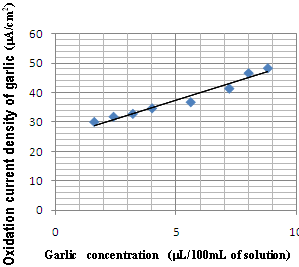
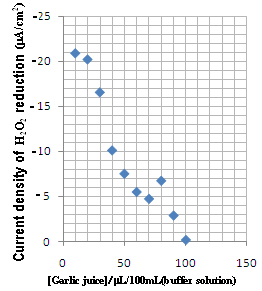
The detection of H 2 O 2 , generated in the silica column, was examined by square wave voltammetry, in the electrochemical sensor. The electrode response was tested for different amounts of H 2 O 2 , in the range from 1µL/100mL (buffer tampon solution) to 100 µ L/100mL (buffer tampon solution). Figure 4 shows some typical square wave voltammetry curves recorded at Cu-CPE electrode. A calibration graph was then constructed from the observed peak currents. The square wave voltammetric response was almost linear dependent on the concentration of H 2 O 2 (Fig. 5). The linear regression analysis gave: Figure 6 shows the garlic juice calibration plots. As can be seen, the garlic oxidation current density increases with concentration. The addition of garlic juice to xanthine solution caused a decrease in H 2 O 2 reduction current signal (Fig. 7). We can conclude that garlic inhibited the reductive effect of H 2 O 2 .
ii. Antioxidant Capacity of Tea
The developed system was also to the determination of the antioxidant capacity of tea. Figure 8 shows the evolution of the oxidation current density of tea with concentration, the peak current have a linear relationship with concentration. For constant concentration of H 2 O 2 , the dependence of square wave voltammetric peak current on the addition of tea in silica column was studied (Fig. 9). The reduction peak of H 2 O 2 decreases in presence of tea.
10. iii. Antioxidant Capacity of Coffee
The working procedure consisted in adding xanthine and coffee to a column containing xanthine oxidase immobilized onto silica. The presence of coffee will induce a decrease of H 2 O 2 concentration, resulting in a decrease of the H 2 O 2 electrochemical sensor (currentdensity). Figure 10 shows evolution of the H 2 O 2 reduction current density with concentration of coffee. ( )
The corresponding antioxidant capacity values, was calculated using the relation:
Where I H2O2 , is the current density due to H 2 O 2 reduction and I H2O2antioxidant sample represent the current density due to antioxidant sample addition. The results are summarized in Table 1.
11. Table. 1
IV.
12. Conclusion
A bioanalytical system for the evaluation of the antioxidant capacity has been developed. The main advantage of the new approach is based on coupling the production of radicals, generated by the xanthinexanthine oxidase enzymatic system, with the electrochemicale sensor, for H 2 O 2 detection. The immobilization of xanthine oxidase (XOD) on the silica increased the sensitivity of the system in comparison with those where the XOD remained in solution. The results obtained show that the proposed system is fast, sensitive and better suited than conventional methods.

![i p = -0.042[H2O2] +-20.59 with a correlation coefficient of 0.9498 c) Antioxidant Capacity Determination i. Garlic juice After drawing the calibration curve relative to a reduction of H 2 O 2 , we introduced into the silica column a solution containing xanthine and the antioxidant considered.](https://medicalresearchjournal.org/index.php/GJMR/article/download/567/version/101836/6-New-Process-Based-on-the-Coupling_html/29443/image-5.png)