1. Effects of Trypanosoma Brucei on Some Electrocardiographic Repolarisation Indices of Dogs
Ajibola, E.S ? , Oyewale, J.O ? , Oke B.O. ? & Rahman S.A. ? Summary-The effect of acute T.brucei infection on some ECG repolarisation indices like QT dispersion (QT D ), heart rate corrected QT dispersion (QT CD ), T wave voltage expressed as T/R, Mean electrical axis (MEA), Heart rate (HR) and plasma potassium concentration was evaluated in dogs.
Ten dogs inoculated with 1 ml of Phosphate buffered saline diluted blood containing 1x10 6 of the federe strain of the parasite had their ECG recorded on weekly basis for three weeks. Plasma potassium concentration was assayed in each dog before the electrocardiogram. Supraventricular arrhythmia, ventricular premature contraction, ventricular tachycardia and various degrees of Atrioventricular blocks were recorded from the eighth day of infection. T. brucei infected dogs had elevated heart rate during the period of infection in all the six leads studied. At various times during the infection, QT and QT C of the infected dogs were significantly lower than the uninfected ones in leads II and AVF. Although the T wave voltage was also increased in the infected dogs, the QT D QT CD , and the MEA were not affected by the infection. The QT D and the QT CD indices of arrhythmic dogs were however found to be significantly lower than in the non-arrhythmic dogs. The serum potassium concentration of the infected dogs was significantly lowered in the first two weeks of infection and then rose to the control level during the third week of infection. Serum potassium concentration in arrhythmic dogs was however not different from the non-arrhythmic ones. The increased heart rate and the shortened QT and QT C width seen during the course of the infection reflect the enhanced state of sympathetic activity and the propensity for arrhythmia in the infected dog.
This result has shown that ECG changes and arrhythmia seen in this study may not strictly reflect structural and functional cardiac involvement but changes in ANS functions coupled with perturbation in electrolyte and the metabolic statues of the infected dogs may have also been implicated. The usefulness of the QT D , QT CD , as a sole marker for the detection of cardiac abnormality is also limited because of the inherent technical problems associated with the measurement of QT.
2. Introduction
anine trypanosomiasis like canine babesiosis, chagas disease and malaria is characterized by myocarditis which often causes arrhythmia ( Dvir et al., 2004; Sprague, 1946; Zhang et al., 1999).
Arrhythmia and conduction block is caused by a decrease in resting membrane potential of the ischemic myocardial cells (Boyden, 1996).
T. brucei infection has been reported to cause gross and microscopic heart lesion starting from the eighth day of infection (Morrison et al., 1983).
Impulse initiation disorders like Ventricular tachycardia and ventricular premature contraction and impulse conduction disturbances like Atrioventricular ventricular block and bundle branch block often characterized T. brucei infection in dogs (Ndungu et al., 1991).
Arrhythmias, especially of Ventricular origin normally affects repolarisation indices like QT dispersion (QT D ), that is, the range of QT interval duration in all measurable ECG leads and corrected QT dispersion (QT CD ) which is the difference between the maximum and minimum heart rate corrected QT intervals.
The usefulness of these indices in canine cardiology and canine trypanosomiasis in particular has not been fully exploited. Previous ECG work done on experimental T. brucei infection in dogs did not explore the effects of this infection on changes in repolarisation indices, heart axis and plasma electrolyte profile (Ndungu et al., 1991).
In this study, electrocardiogram of T. brucei infected dogs will be monitored for changes in cardiac rate rhythms and repolarization indices. And the role of cardiac repolarisation indices as markers of cardiac mortality in canine trypanosomiasis highlighted. This work will apart from addressing the existing knowledge gap between African canine trypanosomiasis and chagas disease, its South American counterpart, it will also provide alternative cardiac indices needed to diagnose and monitor this disease and other related cardiac conditions.
3. II.
4. Animals, Materials and Methods
Ten dogs sourced locally from a local breeder in Abeokuta were used for the study. The dogs were between 3 to 6months old and they weighed between 4?7kg. They were acclimatized for two weeks and screened against trypanosome species and other hemoparasites. All animals that participated in this study showed no evidence of Dirofilariasis or any other cardiac conditions and had received all routine vaccination. Dogs were kept in fly proof kennel, fed twice on commercial dog food (Jojo dog foods, Ikeja) and allowed asses to water ad libitum. This work was conducted in accordance to provisions of the ethical committee of College of Veterinary Medicine, Federal University of Agriculture, Abeokuta.
T. brucei (federe strain) got from Nigerian Veterinary Reseach Institute, Vom, was used for this study. The parasites were preserved by sub-pass aging in donor albino rats. And each dog serving as its own control was inoculated with 1 ml of phosphate buffered saline diluted blood containing 1× 10 6 of the parasites intraperitoneally.
A six-lead (I, II, III, AVR, AVL, AVF) body surface electrocardiogram of dogs placed on standard position was recorded serially before and on days 8, 16, and 24 post infections. The animals were not clipped and contact between skin and electrodes was improved by application of electrode gel. All recordings were made on one of the channels of a four channel universal student Oscillograph (Harvard apparatus, UK) by the same ECG technician. The paper speed was 25mm/sec and the pen sensitivity 10mm=1mV.
The QT interval and the preceding RR interval were measured and averaged in five consecutive P-QRS-T complexes in each lead. These intervals were measured manually to the nearest 0.5mm using calipers and ruler.
The QT interval measured from the beginning of Q to the end of T is defined as the return of T to the isoelectric line (Brooksby et al., 1999). The corrected QT (QTc) was derived with the Fridericia formula; QTc= QT/RR 1/3 (Fridericia, 1920). QT dispersion (QT D ) and the heart rate corrected QT dispersion (QT CD ) were calculated as the difference between the minimum and maximum value of QT and QTc (Dennis et al., 2002).
Mean electrical axis of the heart was determined using the lead graphing method (Edwards, 1993). T wave amplitude was evaluated as T/R ratio (Dvir et al., 2004).
The heart rate was determined by counting the number of cycles (RR interval) in six seconds and multiplying by ten.
Lead II ECG traces obtained from infected and control dogs were analyzed for arrhythmia. The ECG tracings were evaluated for arrhythmia by a panel of cardiologist who were not part of the study.
All data were expressed as Mean ± Standard deviation. Differences within parameters during the course of the disease were evaluated by ANOVA for repeated measures. Statistical significance between the pre-infection control and a value at a particular time point after the infection was determined by paired t-test with bonferoni correction.
The arrhythmia group was compared with the non-arrhythmia group using the t-test for independent sample. P < 0.05 was considered significant. All statistical tests were done using SPSS version 16.
5. III.
6. Results
A total of 240 electrocardiograms were obtained from ten dogs. The QT and the QTc parameters were measurable in all six leads in one hundred and thirty six of the two hundred and forty electrocardiograms.
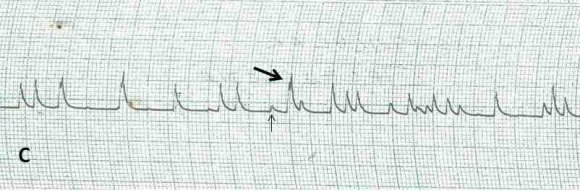
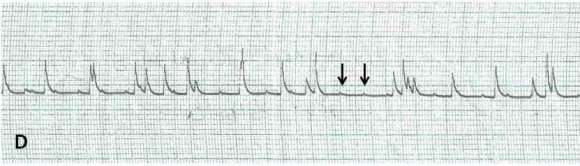
A total of 36 out of 40 lead II electrocardiograms were analyzed for arrhythmia. Four ECG were discarded due to its artefactual content. Twenty electrocardiograms representing 55.5% showed various forms of arrhythmias starting from the 8 th day of infection. Each of the affected electrocardiograms showed at least one form of arrhythmia. Ventricular premature contractions (VPC), ventricular tachycardia (VT), polymorphic ventricular tachycardia (PVT), bundle branch block (LBBB), Atrioventricular blocks (AVB), notched R wave, ST wave slurring (STS), ST wave depression (STD) and sinoventricular rhythm were shown by the dogs at different times during the study( Figure 1-5).
As shown in table 1, the heart rate, T/R voltage and the plasma potassium were significantly affected by the infection but the mean QT D , QT CD , and the MEA, were not affected by the disease progression. Although the mean serum potassium concentration of the infected dogs on the 8 th and 16 th day of infection was significantly lower than in uninfected dogs, the plasma potassium concentration on the 24 th day of infection was significantly higher than on the 8 th day (P?0.01) and on the 16 th day (P?0.01).
When the indices in table 1 were compared between arrhythmic and non-arrhythmic dogs, it was revealed in table 2, that T/R voltage was taller in arrhythmic dogs but the QT CD and QT D were wider in dogs without arrhythmia.
As shown in table 3, T. brucei infection caused a reduction in QT and QTc indices. At days 8, 16, and 24 post-infection, QT was significantly reduced compared to control value. The QTc was however significantly shortened only on days 8 and 24 post-infection.
7. IV.
8. Discussion
The exhibition of various forms of arrhythmia like Ventricular premature contraction, Ventricular tachycardia, Atrioventricular conduction blocks and Supraventricular arrhythmia in the T. brucei infected dogs is consistent with other infections like babesiosis, malaria, and chagas disease (Dvir et al., 2004; Sprague, 1946; Barr et al., 1992). Myocarditis, a common feature of canine trypanosomiasis has been reported by some
9. Global Journal of
workers to provide a suitable substrate for arrhythmia (Boyden, 1996). These arrhythmias are often triggered by enhanced heterogeneity of ventricular repolarisation (Merx et al., 1977; Ahnve and Vallin 1982; Kuo et al., 1983).
Although several workers have reported the association of QT interval prolongation with lethal form of ventricular arrhythmia (ref), the reduced QT and QTc width seen in this study have also been reported to be potentially pro-arrhythmic.
On the surface electrocardiogram, heterogeneity of ventricular repolarisation often manifest as increased QT D , and QT CD (Higham et al., 1992; Day et al., 1990; De-Bruyne et al., 1998). Although the QT D and QT CD were not affected by disease progression, non arrhythmic dogs have a more dispersed QT D and QT CD. This is at variance with Dennis et al., 2002, who reported that QT CD index of arrhythmic animals was insignificantly higher than those without arrhythmia. The extent and location of myocardial are related to the generation of ventricular arrhythmia (Lown et al., 1969; Kuo et al., 1985; Kutz et al., 1994).
Since histopathology was not part of this work, the extent of myocardial damage could not be ascertained. The markedly elevated plasma potassium level on the 24 th day of infection however is an indication of possible myocardial damage (Janse and Witt 1989).
This study similarly to the findings of Barbabosa-Pliego et al (2009), in T. cruzi infection of dogs reported a significantly elevated T/R at a later stage of the infection. When compared with the reference range, the value of T/R either before or after infection was higher than the reference value of T/R?0.25 (Dvir et al., 2004). The increased amplitude of the T wave in this study may be due to hyperkalemia which was noticed at the later part of the infection (El-Sheriff and Turitto 2011). High amplitude T-waves have been linked to hyperkalemia of myocardial infarction (Feldman and Ettinger 1977).
In agreement with Ndungu et al (1991), T. brucei infected dogs in this study showed tachycardia.
The reduced QT and QTc width exhibited by the infected dogs could result from shortened action potential duration and this could consequently increase the heart rate of the infected dogs. Some workers have reported the role of increase cardiac sympathetic activity in myocardial infarction (Esler and Kaye 2000). In T. cruzi infection, rarefaction of the cardiac parasympathetic nerves has been reported (Olivieria, 1985). The increased cardiac sympathetic discharge often seen in myocardial infarction (Jardine et al., 2005) could be the reason for the tachycardia observed in this study.
The Atrioventricular and intramyocardial blocks observed in this study has been previously reported in T. brucei and T. cruzi infections of dogs (Ndungu et al., 1991; Anselmi et al., 1967).
T. brucei infection as seen in this study does not affect the heart's chamber size and axis. Although the MEA of dogs used in this study did not fall within the reference range of 40 °-100 ° as reported by some authors for the specie (Tilley and Larry, 2001;Martin, 2005), they tend to fall within those of humans which have been reported to be between -30 °-90 ° (Fouchet and Gateff 1968). This probably reflected the breed peculiarity of the Nigerian dogs. The arrhythmia observed in some dogs may therefore not necessarily reflect a primary structural myocardial damage but may be a result of metabolic disturbances which often characterize canine trypanosomiasis. Our observation is thus in agreement with Fouchet and Gateff (1968) who earlier reported that axis deviation is not a common finding in African human Trypanosomiasis.
Although the QT interval was read manually in the present study, the repeatability index of the values obtained was high and the values of the QT D and QT CD reported here agrees with those that have been reported previously for normal dogs and those with cardiac conditions (Dennis et al., 2002).
For now, because of the inherent technical problems and the inconsistency associated with measurement of QT index, restraint should be exercised in its use as a marker of cardiac mortality in canine trypanosomiasis.
10. Volume XIV Issue I Version

| © 2014 Global Journals Inc. (US) |