1.
Introduction istacia lentiscus (mastic tree), Family Anacardiaceae, an important medicinal plant is widely distributed in Mediterranean Europe, Morocco and Iberian peninsula and in the west through southern France, Turkey, Iraq and Iran. It has a great medicinal value and already has been used in traditional system of medicines. The pharmacology and medicinal use of mastic is diverse. It has been used in cancer, infection, surgical wound adhesion, and ulcers. Studies also document its use as an antioxidant, antiatherogenic, an insecticide, and anti-inflammatory, anti-microbial and for treatment of hypertension and relief of upper abdominal discomfort, stomachaches, dyspepsia and peptic ulcer (Ahida, et al, 2012, Barra, et. al., 2007, Dedoussis et al., 2004;Lamiri et al., 2001, Bonsignore, et al. 1998, Al-Habbal et al., 1984and Bentley et al., 1980). In addition to its medicinal usage, it has been re-evaluated as a flavoring in chewing gum (Fernandez et al., 2000).
Phytochemicals are chemical compounds that occur naturally in plants. study done by Kumar and Singh (1976) refereed to that phytochemicals are secondary metabolites and are often found in stems, roots, barks, leaves, flowers, fruits and seeds. Common phytochemicals found in plants include tannins, phlobatannins, quinines, alkaloids, saponins, flavonoids, steroids, terpenoids, cardiac glycosides, sugar. They are having potential to affect diseases such as cancer, gout, rheumatism, arthritis (Berboucha et al., 2009;Dimaset al., 2009;Peksel, 2008, Balan, etal, 2007and Baytop, 1999). Cortisone is a steroid hormone and is used for a variety of ailments. These include endocrine disorders, rheumatic disorders, collagen diseases, dermatologic diseases, allergic states, ophthalmic diseases, respiratory diseases, hematologic disorders, neoplastic diseases, edematous diseases.
Antioxidant -Most phytochemicals have antioxidant activity and protect the cells against oxidative damage and reduce the risk of developing certain types of cancer activity includes: flavonoids, polyphenols.
Anthracene is glycosidic compounds of formula C 14 H 10 .
Anthracene is converted mainly to anthroquinone, a precursor to dyes (Gerd, et al., 2006). It uses in pesticides (Agency for Toxic Substances, 1999). Study by Dembitsky VM (2005) reported that Anthracene and its derivatives, isolated from and identified in plants and microorganisms that demonstrate different biological activities. They are of great interest, especially for the medicinal and/or pharmaceutical industries. These biologically active natural surfactants are good prospects for the future chemical preparation of compounds useful as antioxidant, anticancer, antimicrobial, and anti-bacterial agents (Dembitsky, 2005). This study was conducted to determine some photochemical present in Pistacia lentiscus shoot extract. We also evaluated its mineral composition.
2. II.
Material and Methods a) Plant material Shoot of P. lentiscus was collected around from Msallata (northwestern part of Libya). Plants were identified according to (Ali, et al., 1977 ii. Saponins Test: (Froth test) 1g of the sample was weighed into a conical flask in 10ml of sterile distilled water was added and boiled for 5 min. The mixture was filtered and 2.5ml of the filtrate was added to 10ml of sterile distilled water in a test tube. The test tube was shaken vigorously for about 30 second. It was then allowed to stand for half an hour. Honeycomb froth indicated the presence of saponins.
iii. FlavonoidsTest Flavonoids was determined by placing 5ml of the solvent extracts into a test tube and few pieces of magnesium chips were added, followed by concentrated hydrochloric was added drop wise. Appearance of Reddish colouration demonstrated the presence of Flavonoids. iv. Proteins Test 1ml of plant extracts was placed into a test tube then 4ml of foline reagent was add. Appearance of blue colouration demonstrated the presence of proteins.
v. Phenol Test 2ml of extract was added to 2ml of ferric chloride solution (FeCl 3 ); a deep bluish green solution is formed with presence of phenols.
vi. Phlobatannin Test 3ml of hydrochloride acid and 2ml of solvent extract were placed into a clean test tube and the tube was heated for about 10 minutes. Reddish green coloration indicates the presence of phlobatannins.
vii. Hydrolysable tannins 4 ml of the extract was placed and shaken in a test tube. 4ml of 10% ammonia solution was added. Formation of an emulsion on shaking indicated the presence of hydrolysable tannins.
viii. Tannins Test 3ml of sample extract was boiled in 50ml distilled water, warmed and filtered. A portion of the filtrate diluted with sterile distilled water in a ratio of 1:4 and 3 drop of 10% ferric chloride solution added. Green colour indicates the presence of tannins.
ix. Volite Oil Test 2.0ml of extract solution was mixed with 0.1 ml dilute sodium hydroxide and a small quantity of dilute HCl. A white precipitate was formed with volatile oils.
x. Anthracanens test 3g of sample powder was mixed with 4ml of ammonia solution, heated for 15 min. Red color indicated the presence of Anthracanens.
xi. Glycosides Test 25 ml of dilute sulphuric acid was added to 5 ml of the extract in a test tube and boiled for 15 minutes, cooled and neutralized with 10% NaOH, and then 5 ml of Fehling solution A and B was added. A brick red precipitate of reducing sugar indicates the presence of glycosides.
3. b. IR Spectra
A small amount of each extraction was placed on the diamond attenuated total reflectance (ATR) crystal of the Agilent Cary 630 ATR-FTIR analyzer. The samples were pressed against the diamond crystal using the attached pressure clamp. FTIR spectra were acquired in less than 30 seconds. The spectra of reference storied (cortisone) samples were automatically stored in a dedicated spectral database. The spectra of extractions samples were then analyzed using an automated output pass/fail or percentage (%) similarity. 10 ug of pure cortisone was dissolved in different solvent (water, petroleum ether, hexan and ethyl acetate, respectively) and were used for comparison c. UV-Visible spectrophotometer 5g of leaves and stem was extracted with 125ml of ethyl acetate and ethanol (individually). Cortisone was detected in sample by UV-Visible spectrophotometer (Agilent Cary 60 UV-Vis). Cortisone standard was dissolved in ethyl acetate or ethanol then loaded for comparison. e) Thin Layer Chromatography (TLC) i. TLC analysis Chromatography plates were prepared using silica Gel, 60 F254 TLC Aluminum Sheet 20x20cm Merck-Germany. The samples were spotted on the plates with graduated capillary tubes (5 µL). The standard of cortisone was dissolved in different solvents (water, petroleum ether, hexan and finally ethyl acetate) then spotted for comparison. Toluene-ethyl acetatediethyl-amine (14:2:2) were used as mobile phase.
ii. Detection Cortisone band was observed as follows: * Without treatment using UV (254-356nm). *Universal detection reagents: using both concentrated sulphoric acid and iodine vapor from crystal then comparing its rate of flow (R f ) value with standard.
4. f) Mineral Compounds Analysis
The dried plant was wet oxidized and the elements were determined by Atomic Absorption Spectrophotometer (Perkin-Elmer model 403, Norwalk Ct, USA). The minerals were reported in ppm. The minerals include sodium, potassium, calcium, magnesium, iron, copper and Zn, Pb, Cd and Cr. Values for, Fe, Cu and Mg were read on Atomic Absorption Spectrophotometer (180-30 Hitachi) after standardizing with respective elements (American Association of Cereal Chemists, 1984).
5. III.
6. Results and Discussion
7. a) Phytochemicals analysis
The results illustrated in Table I; the phytochemical analysis conducted on water and ethylacetate shoot extracts of P. lentiscus revealed the presence of some bioactive components such as alkaloids, tannins, hydrolysable tannins, phlobatannins, phenol, volatile oil, saponins, glycosides, flavonoids, protein and Anthracanens. 2.
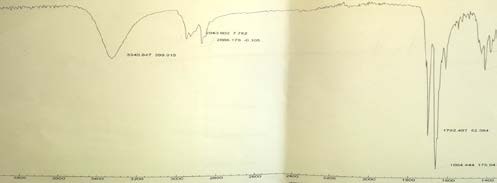
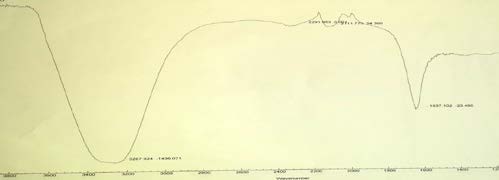
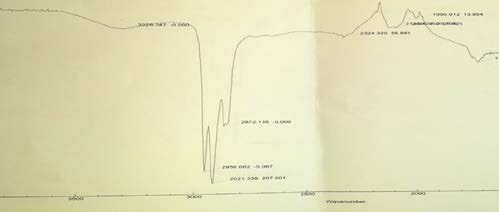
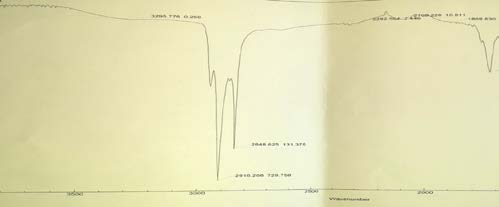
8. Table 2 : Peak of cortisone in FT-IR Spectra
The result also illustrated that water extract of P. lentiscus did not reveal the presence of cortisone, where infrared absorption peak at 3267.924 and unlike pure cortisone (standard).
9. ii. UV-Visible Spectrophotometer
Ethanol and ethyl acetate extract of P. lentiscus shoot were analyzed for cortisone detection using UV- iii. Thin Layer Chromatography (TLC) Standard cortisone and P. lentiscus shoot extracts (with petroleum ether, haxen and ethyl acetate, individually) were spotted on TLC plates then developed using 18Toluene: 2 ethyl acetate: 2 diethyl amine as a solvent. Spots were detected using iodine vapor, UV and concentrated sulphoric acid (Heftmann, et al., 1966). The qualitative evaluation of the plate was done by determining the migrating behavior of the separated substances given in the form of RF value (Mendham, et. al., 2002). Result of this study, as shown in Table 3 and Fig. 2, indicated that there was match between standard cortisone and plant extracts in terms of spots number and RF values.
Table 3 : TLC results of P. lentiscus shoot extracts and standard TLC profiling of P. lentiscus shoot extracts in different solvent system confirms the presence of diverse group of phytochemicals (steroids). Different R F values of the compound also reflect an idea about their polarity. This information will help in selection of appropriate solvent system for further separation of compound from P. lentiscus shoot extract (Das Talukdar et al., 2010).
10. c) Mineral components
Investigated elements were chosen (Na, Zn, Fe, K, Cu, Ca, Mg, Cd, Cr, and Pb) according to their role and importance in many biological mechanisms. Quantitative determinations were made of the mentioned elements in the shoots of P. Lentiscus. The composition in major and minor minerals of the shoots of P. lentiscus is detailed in Fig. 3 and 4. The importance of mineral composition is due to their nutritional properties and beneficial health effects, as well as their meeting of dietary guidelines required for a healthy diet (Welna et al., 2008). Results of this study revealed to that Potassium had the highest content of (15.44ppm) dry weights this result in agreement with study done by (Aouinti et al., 2014). The level of Ca in the shoots of P.lentiscus in this work was found to be higher (2.66 ppm) dry weight compared to Na (0.25 ppm). Calcium is the major component of bone and assists in teeth development (Brody, 1994). constitutive element of chlorophyll, so that its highest concentration was found in leaves (Aouinti et al., 2014). In this study, the average concentration of Magnesium in the P.lentiscus shoot was 2.41 ppm.
Iron functions as hemoglobin in the transport of oxygen. Iron functions as essential component of enzymes involved in biological oxidation such as cytochromes (Malhotra, 1989). It is an important constituent of succinate dehydrogenase as well as a part of the haeme of haemoglobin (Hb) and the cytochromes (Chandra, 1990). Though it is an essential element, excess intake can lead to iron toxicity and can damage lipids and proteins (Bothwell, et al., 1979 andFraga, et al., 2002).
Result of this study revealed to that shoots of P. lentiscus are reach in iron with a concentration of 0.63 ppm. This result is consistent with the (Aouinti et al.
11. 2014).
Cupper and Lead has been detected in the shoot of P. lentiscus with concentration of 0.14ppm.
Cupper is component of many redox and ligninbiosynthetic enzymes. In plant, its deficiency symptoms include Chlorosis, dead spots in leaves, stunted growth, terminal buds die, necrosis in young leaves (Soetan, et al., 2010). There are also inter-relationships of iron, copper and cobalt (in vitamin B12) in hemoglobin synthesis and red blood cell formation (Hays, et. al., 1985). Lead is best known for its toxicological properties beef liver and also in RNA preparations (Uppala et al., 2005). It could play a role in maintaining the configuration of the RNA molecule, because Cr has been shown to be particularly effective as a cross-linking agent for collagen (Eastmond et al., 2008).
IV.
12. Conclusion
The phytochemical tests performed on the shoot extracts of P. lentiscus shows the presence of alkaloids, tannins, hydrolysable tannins, phlobatannins, phenol, volatile oil, saponins, glycosides, flavonoids, , protein and Anthracanens. The present study revealed the presence of cortisone in P.lentiscus shoot extracts which were confirmed by various techniques studies. Since cortisone contains a wide range of medicine and pharmacological properties, they can be exploited more storied in future for further studies.
13. V.
Magnesium is not only essential, but it is also a Zinc is distributed widely in plant and animal tissues and occurs in all living cells. Zn dependent enzymes are involved in macronutrient metabolism and cell replication (Hays, et al., 1985). In humans, deficiency disease or symptoms include hypogonadism, growth failure, impaired wound healing, and parenteral nutrition (Murray et al., 2000).
Cadmium and chromium have detected in the shoots of P. Lentiscus with low concentration (0.01 and 0.02 respectively. The possible effects of the general population of long term, low level exposure to cadmium have been of concern recently (Asagba, 2009), because cadmium readily distributed in tissues after exposure and it inhibits antioxidant enzymes (Chater et al.,2009;Asagba and Eriyamremu, 2007;Bagchi et al.,1996;Gupta et al., 1991) (Macrae, et al, 1993) there are increased in depressives and schizophrenics but reduced in manic patients (Stanley, et al., 2002).
Lead is an ubiquitous environmental and industrial pollutant that has been detected in every facet of environmental and biological systems.
Figueiredo-Pereira et al., 1998). Chromium is an essential element for animals and humans (Frieden, 1984). It has been found in nucleoproteins isolated from Pharma cological Properties. International Journal of Phar-macy and Pharmaceutical Sciences. Vol (4):1-20.
14. American Association of Cereal Chemists (AACC).
Method 08-01. The Association St.Paul, M.N. 1984. 5. Aouinti, F., Zidane1, H. Tahri1, M., Wathelet, P.j.and El Bachiri1, A. (2014).Pistacia lentiscus L. from Eastern Morocco. J. Mater. Environ. Sci. 5 (1)

| b) Preparation of plant extracts |
| Leaves and parts of stem (10gm) were |
| subjected to sohxlet extraction using 200ml of water and |
| ethyl-acetate (individually) as solvents. Samples kept for |
| 6 hours at 90?C for water and 70?C for Ethyl-acetate. |
| c) Phytochemical tests |
| Plant extracts, of both solvent water and |
| ethylactate, were screened for the presence of |
| biologically active compounds like alkaloids, flavanoids, |
| Protenis, Phenol, Phlobatannin, Volte oil, Hydrolysable |
| Tannins, Tannins, Saponins, Glycosides, Anthracanens |
| and Cortisone. |
| d) Procedure for Phytochemical Analysis |
| i. Alkaloid Test |
| Equal volumes of the solvent extract (5ml) and |
| the Wagner's reagent, described by Imohiosen et al.( |
| 2014), were placed into a clean test tube and observed |
| for some minutes. The presence of alkaloid was |
| indicated by a brown precipitate. |
| 6-Volite Oil | 0.1 % NaOH + HCl | A+ B+ | layer oil |
| 7-Hydrolysable tannins | Amonia solution | A+ B++ | Formati on of an emulsion |
| 8-Tannin | 10% ferric chloride, 50ml distilled water heating 30 min. | A+ B++ | Green precipita te |
| 9-Saponins | Froth test | A+ B+ | Honeyc omb froth |
| 10-Glycosides | Fehlange | A+ B+ | red |
| 11-Anthra -canens | Amonia solution | A+ B+ | red |