1. Introduction
n 2008 burns accounted for the majority (19%) of all non-transport injury deaths in South Africa [1], and are a significant cause of paediatric morbidity [2] and mortality [3]. The costs associated with the treatment of burns are considered to be large [4]. Due to resource constraints in South Africa, treatment options that can ease this burden without compromising clinical outcomes are starting to be explored [5]. The need for daily dressing changes has not only been shown to increase the cost of burn wound care in some instances, but is also associated with pain and trauma, which is of particular importance in the paediatric patient [6].
A standard of care (SOC) depends on the number of factors, including experience, expertise and resource availability [7]. Our existing SOC for partial thickness scald or fire burn consists of initial cleaning and/or debridement in the ward or theatre, followed by the daily application of silver sulphadiazine dressing. The adoption of this particular SOC may not represent the most clinically appropriate or efficient form of resource allocation.
Biobrane is a biosynthetic wound dressing, and is becoming more widely used in the management of burns, particularly partial thickness burns in paediatric patients [8]. When used appropriately, it has been shown to offer significant advantages over more conventional therapy , including decreased time to heal [9], decreased length of stay [9][10][11], fewer dressing changes [10] and decreased costs [10,11]. The removal of necrotic skin, tissue and/or infectious materials is the primary goal in the initial treatment of burn wounds [12]. While surgical debridement is considered the goal standard, it has been suggested that a hydrosurgery system such as Versajet may have advantages over traditional escharectomy in burn patients [12]. Versajet utilises a fluid jet under high pressure, and has been shown to be effective at cleaning and debriding superficial and intermediate depth burn wound prior to the application dressings like Biobrane [13]. A number of institutions have adopted nanocrystalline silver (NC) dressing as the SOC for firstline topical prophylactic treatment of burn-wound infectious [7,14]. Acticoat is a NC dressing that can stay in place for up to 72 hours.
Objectives: The primary objective was to compare the time to healing of partial thickness burn wounds treated with Versajet, Biobrane and Acticoat to conventional therapy. Methods: A randomised, controlled, prospective study was undertaken.
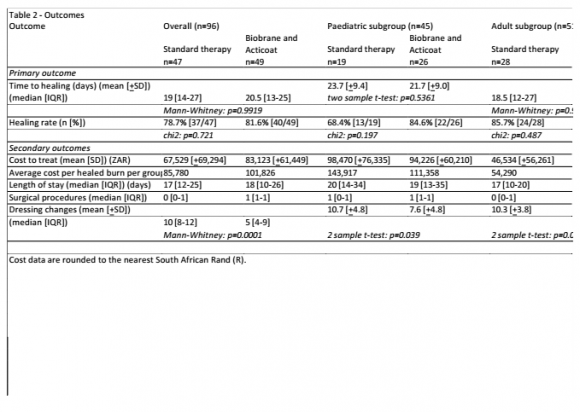
Results: One hundred and twenty one patients were randomised, and 96 were analysed. Median time to healing was slightly shorter for Biobrane-treated patients in the pediatric sub-group (21.7 [±9.0] versus 23.7 [±9.4], p=0.5361), and slightly longer in the adult sub-group (19 [12][13][14][15][16][17][18][19][20][21][22][23][24][25] versus 18.5 [12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27], p=09695). Healing rates were higher for Biobrane-treated patients in the paediatric sub-group (84.6% versus 68.4%, p=0.197), and lower in the adult subgroup (78.3% versus 85.7%, p=0.487). . The absolute risk reduction (ARR) for Biobrane-treated patients in the paediatric sub-group was 0.16 (16%), yielding a number-needed-to-treat (NNT) of 6. The median number of dressing changes was significantly lower for Biobrane group in the adult and paediatric population (6.2 [±3.6], p=0.0003; and 7.6 [±4.8] versus 10.7 [=4.8], p=0.039). The mean cost per healed burn for Biobrane group was lower in the paediatric population (R111, 385 versus R143,917), and higher in the adult population (R54, 290 versus R90.175).
We hypothesized that the advantages described for Biobrane in the treatment for partial thickness burns combined with the prophylactic antiinfective properties of a NC dressing might represent an effective utilization of resources, and set out to investigate this in a randomised controlled study. The primary objective of our study was to compare the time to healing of partial thickness burn wounds treated with Versajet, Biobrane and Acticoat to standard therapy over a period of up to 6 weeks. Secondary objectives were to compare costs, length of inpatient stay, and number of surgical procedures and dressing changes performed in the above groups.
II.
2. Methods
This study was carried out as per the principles laid out in the Declaration of Helsinki and subsequent amendments thereto. It was approved by the Human Research Ethics Committee: (Medical) of the University of the Witswaterstrand (090203). Informed consent was obtained for patients.
All eligible patients between 1 and 60 years of age requiring admission to hospital for partial thickness scald or five burns covering greater than 10% and less than or equal to 40% of total body surface (TBSA), with an estimated healing time of between and 21 days, were invited to participate in the trail.
Following stabilization, patients were randomized Standard therapy or Biobrane using the sealed envelope technique. To ensure broadly comparable patient groups with regard to the % TBSA of the burn, the randomization was stratified according to the estimate initial % TBSA of the burn. In those patients with more than 1 discrete burn, the largest burn was considered the reference wound.
An assumption was made regarding mean time to healing in the 2 groups, and the associate standard deviations, as information was lacking regarding time to healing and the size of the difference Standard therapy and Biobrane. The sample size calculation (conducted using STATA software) resulted in a required sample size of 80 patients.
Patients randomised to Standard therapy had their wounds cleaned and dressed in theatre or in the ward/dressing station, at the discretion of the investigator. A silver sulfadiazene dressing was applied, covered secondarily with abdominal swabs or plastic gauze, and kept in position with a crepe bandage. Daily dressing took place until such time that the reference wound had healed or 6 weeks had passed, in hospital or as an out-patient following discharge. Wound healing was defined as complete epithelialisation in the absence of drainage.
Patients randomised to Biobrane were taken to theatre, where their wounds were cleaned and debrided with Versajet. Biobrane was applied and help in place using staples, tape, sutures or skin closure strips. Acticoat was applied damped over the Biobrane, covered secondarily with Melolin and kept in position with crepe bandage. The Acticoat was removed on Day 3, and re-applied at the discretion of the investigator if secure Biobrane adhesion had not been obtained. If further Acticoat was not required, the wounds were covered with a crepe dressing. On Day 6, staples or sutures are removed at the discretion of the investigator. If there was no adherence, all Biobrane was removed and the burn wounds were treated according to protocol. If the Biobrane was fully adherent or showed signs of purulent pockets, the areas of non-adherence were trimmed out and covered with Acticoat, Melolin and a crepe bandage. Wounds were assessed every 3 days after and Acticoat dressing were changed if in situ, until such time that the wound bed appeared healthy and granulating, at which point the wounds and the remaining adhered Biobrane were covered with a crepe dressing. When the Biobrane stared to lift, loose edges were trimmed. This process was repeated every 3 days until such time as the reference wound had healed or 6 weeks had passed.
All patients were evaluated at each dressing change while in hospital, and at out-patients visits hereafter. For each dressing change and theatre visit related to the reference wound, burn-specific and resource-specific information was recorded.
The primary endpoint was the average time to heal the reference burn per group divided by the number of healed reference burns per group. Secondary endpoints for comparison between the 2 study groups included the average cost per healed burn per group, defined as the total cost of treatment for all patients per group at 6 weeks divided by the total number of healed burns per group at 6 weeks, the average length of who in-patient stay per group, with wound healing used as a proxy for discharge date in those patients who couldn't not be discharged due to poor socio-economic circumstances, and the number of surgical procedures and dressing changes performed per group. For numeric variables, medians and inter-quartile ranges were cited for data that was not normally distributed. Cost data was always represented by the average cost per patient.
The cost analysis was performed from a public health sector perspective. The study aimed to quantify direct costs associated with using an ingredients approach. Cost categories included: dressing and other materials, nursing time, surgeon and anesthetist time, theatre levels of care, out-patient treatments, blood products, investigation and medications. Indirect costs, including those pertaining to the patients, and their families and caregivers were not included. South African public sector costs excluding value-added tax (VAT) for the financial year ending February 2011 were used, and
3. III. Results
Recruitment commenced in April 2009. Following a scheduled interim analysis, the sample size was increased to 100 patients. Due to loss to follow-up for a variety of reasons, a subsequent request to increase sample size from 100 to 120 to allow for analysis on sample close to 100 patients was made and granted. Patient enrolment was compelled in January 2012. Study subjects were analysed on a per protocol basis and only patients that completed the study as per protocol were included. In total 121 patients were randomized, of which 96 were analysed. Participant flow, including the protocol violations and reasons for exclusion from analysis, are summarised in Figure 1.
The median age of all the subjects was lower in Biobrane group (6.6[3.4-34.2] versus 23.2[2.3-25.4] years); however this difference was not found to be statistically significant (p=0.0984), and was not apparent in the paediatric (defined in our institution as less than 10 years) and adult sub-groups. Additional baseline characteristics are summarized in Table 1, and are comparable between the 2 groups.
Table 2 illustrates results for primary and secondary outcome measures, and includes a subgroup analysis of both children and adults. The median time to healing was slightly longer in the Biobrane therapy group overall (20.5 [13][14][15][16][17][18][19][20][21][22][23][24][25] versus 19[14-27], p=0.9919) and in the adult sub-group (19 [12][13][14][15][16][17][18][19][20][21][22][23][24][25] versus 18.5 [12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27], p=0.9695), and slightly shorter in the paediatric sub-group (21.7[±9.0] versus 23.7[±9.4], p=0.5361). Healing rates were higher in Biobrane-treated patients overall (81.6% versus 78.7%, p=0.877) and in the paediatric sub-group (84.6%versus 68.4%, p=0.197). Healing rates were lower for Biobranetreated patients in the adult sub-group (78.3% versus 85.7%, p=0.487). The absolute risk reduction (ARR) in the paediatric sub-group for Biobrane-treated patients was 0.16 (16%), yielding a number-needed-to-treat (NNT) of 6.
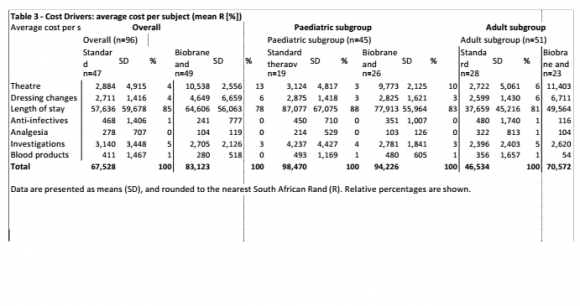
The median LOS was slightly longer for Biobrane group overall, (18 [10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26] versus 17 [12][13][14][15][16][17][18][19][20][21][22][23][24][25], p=0.8978), but the median was shorter in both the adult and paediatric sub-groups (16 [9][10][11][12][13][14][15][16][17][18][19][20][21][22] versus 17 [10][11][12][13][14][15][16][17][18][19][20], p=0.6700; and 19 versus 20 [14-34], p=0.7685). The median LOS prior to the reference wound being deemed suitable for management on an outpatient basis was shorter for the Biobrane group overall, (10 [5-23] versus 16 [12][13][14][15][16][17][18][19][20][21][22][23], p=0.0417), and also in the adult and paediatric sub-groups (9[5-21] versus 15.5 [9.5-20], p=0.0000); and 12.5 [7.24] versus 20 [14-34], p=0.08). As shown in Table 2, the median number of surgical procedures was higher for the Biobrane group than the Standard group (1[1-1] versus changes was lower for the Biobrane group overall (5[4-9] versus 10 [8-12], p=0.0001), and also in the adult population (6.2 [±3.6] The mean cost per healed burn was higher for the Biobrane group overall (R85,780 versus R101,826) and in the adult subgroup (R54,290 versus R90,175). In children the average cost to heal the burn was lower in the Biobrane group (R111,358 versus R143,917). Length of stay was the most significant cost driver across all groups, accounting for between 70 and 80 percent of all costs associated with burn wound management. (Table 3). The mean analgesic costs in the Biobrane-treated subjects were at least half those for the Standard therapy-treated subjects for all patients, including children and adults. The mean anti-infective costs were lower in the Biobrane-treated subjects overall (R241 versus R468, in children (R351 versus R450), in adults (R116 versus R480).
Table 4 shows a further sub-analysis by burn size. Observed differences in time to heal and healing rates did not reach statistical significance. In the large burn paediatric sub-group, the average cost per healed burn for the Biobrane group was less than half that of the Standard group (R146,974 versus R299,461).
IV.
4. Discussion
Much of the published literature looking at Biobrane has conducted in a paediatric setting [7,10]. We observed a small decrease in time to healing in Biobrane-treated patients in our paediatric sub-group, although this did not reach significance as reported previously [9]. The increased healing rates associated with Biobrane in the paediatric subgroup translated into 16% risk reduction in the development of an unhealed burn. Increased healing rates in our paediatric subgroup also contributed to the observed decreased decrease in cost per healed burn, another important consideration in our environment. This cost advantage was particularly noted in the large burn subgroup. Although LOS was decreased for children treated with Biobrane, this decrease did not reach significance and was not as large as might have been expected from other published studies. This is due in part to the challenging socio-economic circumstances facing many of our patients. Under normal circumstance we would discharge patients to out-patients follow-up as soon as the rate of burn wound healing allowed it, and certainly in patients where dressing changes were required to be performed every 3 days (as opposed to on a daily basis). Unfortunately during the course of the study we found that we were unable to discharge a number of these patients for follow up in the community, for reasons purely non-medical, and solely related to lack of social support structures. For this reason, we started to capture the date on which the reference wound was deemed suitable for management on an out-patient basis. Biobrane would appear to offer a significant advantage over Standard therapy in this regard in paediatric patients and we would therefore aim to record this parameter formally in future-planned studies.
The time to suitability for management of the reference wound on an out-patient basis was similarly significantly shorter for Biobrane-treated patients in the adult subgroup, this did not translate into a reduced or even comparable LOS cost when compared to Standard therapy-treated patients.
The fact that this group had higher ICU and high care costs may be related to the requirement for general anaesthetic for initial Versajet debridement, although the same trend was not observed in the paediatric population. A recent retrospective analysis suggests that in adults Biobrane may be better suited to extensive superficial burns, rather than smaller mid dermal or mixed depth burns [15]. Identifying burn depth with certainty on admission is an ongoing challenge to the burns surgeon -a number of burns we believed to be partial thickness on admission later revealed themselves to be full thickness, necessitating alternate treatment in both groups.
The increased mean number of surgical procedures for the Biobrane group was expected, given that the protocol called for all initial dressing changes to be done in theatre for this group. The significantly decreased number of dressing changes for the Biobrane group is in line with findings of other studies [10]. The associated decreased trauma is important, particularly for the paediatric population. Although not a significant cost-driver, the markedly reduced analgesic costs in all Biobrane-treated patients provide further evidence of this reduction in discomfort.
Pseudomonas infection is always of concern in the burns unit, and was noted on more than one occasion over the course of our trail. Although not the focus of our study, we did note that Biobrane use is incompatible with Pseudomonas infection, and a high level of suspicion for this organism is warranted in cases of non-adherence.
V.
5. Conclusion
Although Biobrane and Acticoat did not lead to a significant decrease in time to healing when compared with Standard therapy, we believe the significant reduction in dressing changes observed for this regime to be important, particularly in children. Together cost reduction to treat and heal partial thickness burns, we recommend Biobrane and Acticoat be considered first line therapy in children with partial thickness burns, and have adapted our Standard of Care accordingly. In adults, although a significant decrease in dressing changes was observed, the increased costs associated considered only in carefully selected patients. Deep partial thickness burns has a poor outcome when Biobrane is used and prone to pseudomonas infection thus a superficial partial thickness burns will be more beneficial using this modern regime.We have concluded as well that reduction in analgesics and antibiotics used in Biobrane group reflects respectively reduction in pain and infection overall for these patients. Any new treatment for burns must always take in account the socioeconomic condition of the patient environment. In the future we need to evaluate if all patients need to be debrided in operating room prior to application of skin substitutes.
6. VI.
7. Conflict of Interest Statements
No conflict of interest in the study.

![South Africa Rands (R). Costs were 0 [0-1], p=0.0002). Conversely, the number of dressing](https://medicalresearchjournal.org/index.php/GJMR/article/download/830/version/100439/2-A-Prospective-Single-Centre_html/7363/image-3.png)