1. I. Introduction
ustained and controlled delivery of drugs to the ocular tissues continue to remain a major objective for formulation scientists and engineers in light of the emergence of more potent drugs and biological response modifications with limited biological half-lives. In ophthalmic drug delivery, in the front of the eye, the major hurdles of optimum drug bioavailability include rapid turnover, lacrimal drainage, reflex blinking and drug dilution by tears. Another physiological constraint is the limited permeability of cornea resulting into low absorption of ophthalmic drugs. A major portion of the administered dose drains into the nasolacrimal duct and thus can cause unwanted systemic side effects. Additionally, the rapid elimination of the drug through the punctum results in a short duration of the therapeutic effect resulting in a frequent dosing regimen.
A significant challenge for the formulation is to circumvent these protective barriers of the eye without causing permanent damage to the tissue. Due to these physiological and anatomical barriers, only a very small fraction of the drug, usually 1-5% or even less of the instilled dose, is effectively absorbed. Frequent instillations of eye care solution are necessary to maintain a therapeutic drug level in the tear film or at the site of action. The frequent use of ophthalmic solutions may induce toxic side effects and cellular damage at the ocular surface. Moreover, once-a-day or twice-a-day formulations would like to improve patient compliance.
2. a) Materials
Naphazoline hydrochloride, Pheniramine maleate, HPMC E4M, NaCMC, Benzalkonium chloride, Sodium chloride, NaoH.
3. II. Methods
To prepare viscous eye care solution, different concentrations of HPMC E4M and NaCMC as per 3 2 factorial design were used. In order to obtain clear viscous solutions, accurately weighed HPMC E4M and NaCMC were dispersed and thoroughly hydrated in about 30 ml of the required amount of WFI. The dispersion was vigorously stirred and heated to 80-90°C, until the solution became clear. Then add weighed quantity of Sodium Chloride, Disodium Edetate, Disodium Hydrogen Phosphate, Sodium Hydroxide and at last add weighed quantity of drugs and Benzalkonium Chloride in about 40-50% of the required amount of WFI. Add this mixture to the dispersion of polymers while stirring. Adequate WFI was then added to obtain the required volume. The viscous solutions prepared with the excipients were sterilized at 121°C in autoclave for 20 min. Afterward, aqueous solutions were sterilized by filtration through 0.22 ?m sterile filter. The same formulation was prepared into simulated tear fluid in place of WFI.
4. III. Experimental Design
Experimental designs are frequently used to establish an empirical relationship in terms of a mathematical model between dependent variables, and a number of factors or independent variables. To study all the possible combinations of all factors at all levels, a S Volume XV Issue II Version I
Year 2 015 ( D D D D ) Btwo factor, three level full factorial design was constructed and conducted in a fully randomized order. The experimental design consists of total 9 experiments. The concentration of HPMC E4M (X 1 ) and concentration of NaCMC (X 2 ) was selected as formulation (independent) variables. The factor levels were chosen from the knowledge obtained from the preliminary studies. All other formulation and process variables were kept invariant throughout the study. Table 2 Summarizes the 9 experimental runs studied and their factor combinations and translation of the coded levels to the experimental units employed during the study. The viscosity (Y 1 ), Mucoadhesion index (Y 2 ), cumulative percentage drug released after 8 hrs. (Y 3 ) were studied as response variables (dependent variables).Design-Expert software (V.8.0.7.1, Stat-Ease Inc.) was used for the generation of the mathematical models. iv. Osmolarity The osmolarity of optimized sterilized viscous eye care solution was determined with a vapor pressure osmometer at room temperature.
5. v. Mucoadhesion Tes
Mucin dispersion method was used to measure mucoadhesion index. Mucin Dispersion (MUC): MUC (15%w/v) was prepared by dispersing required amount of Mucin powder -into phosphate buffer (pH 7.4 ) and kept on magnetic stirrer at 600 rpm for 24 hrs for complete hydration and measured it viscosity (?m). To study the mucoadhesive interaction, 5 ml of polymer dispersion (?p) and 15 ml of MUC was mixed and determined its viscosity (?t) at 37°±1°C at shear rates D of 12.5, 25, 50, and 100 s -1 . An interaction between polymer and mucin should be seen as a synergistic effect in the rheological properties, which means that the rheological response of the Polymer/MUC mixture should be larger than the sum of the rheological responses of the single components polymer and mucin. Therefore, it is essential to rheologically characterise the single components as well as the polymer/MUC mixture.
Trial No. Coded Factor X 1 Conc.of HPMC E4M (%w/v) X 2 Conc. Of NaCMC (%w/v) F1 1 1 F2 1 0 F3 1 -1 F4 0 1 F5 0 0 F6 0 -1 F7 -1 1 F8 -1 0 F9 -1 -1vi. Sterilization Study Membrane filter of 0.2-?m porosity was used for sterilization by filtration in ?m aseptic area. The suitability of both methods of sterilization in the present study was tested by determining drug content of representative eye solution formulation of (aqueous based) before and after subjecting to sterilization by the respective method. Aqueous base solutions were prepared with 1 % drug and other excipients filled in vials and sealed. These vials were autoclaved for 20 minutes. In some other vials, solutions were filled by filtration through 0.2?m Millex, Millipore filter.
6. vii. Sterility testing
The sterility testing of the viscous eye care solution was performed for the aerobic, anaerobic bacteria and fungi by using alternative thioglycolate medium (ATGM) and soyabean casein digest medium (SBCD). The positive control (growth promotion), negative control (sterlity) test was also conducted. Bacillus subtilis was used as a test organism in the case of aerobic bacteria test. Bacterio desvulgatus was used as a test organism in case of anaerobic bacteria test and candida albicans in fungi test. Incubation was carried in all cases and growth was checked. All the samples were inoculated separately in to ATGM and SBCD media and incubated at 35°C and 20-25°C, respectively for 7 days. Similarly unsterilized samples of films were also inoculated separately in to ATGM and SBCD media and incubated at 35°C and 20-25°C, respectively for 7 days. A control evaluation was also carried out.
viii. Antimicrobial Test The test was performed according to USP. Cultures of bacteria [Escherichia coli (ATCC 4352), Pseudomonas aeruginosa (ATCC 9027), Staphylococcus aureus (ATCC 6538)] and fungi [Aspergillus niger (ATCC 16404) and Candida albicans (ATCC 10231)] were grown on solid agar media and soyabean casein digest media respectively. The test microorganism cultures were diluted with sterile WFI to obtain 10 -6 CFU/ml as per USP. All the cultures were transferred into 5 test tubes containing 10ml prepared eye drops(solution) and 0.1% prepared cultures in each. Initial counts were noted. These solutions were poured into petri plate containing Soya bean Casein Digest Agar Medium for bacterial cultures and Sabouraud Glucose Agar Medium for fungi. Keep them into incubator at 32.5±2.5ºC and 22.5±2.5ºC for bacteria and fungi respectively. Record the numbers of colony of microorganism at 7 th , 14 th and 28 th day. Check the criteria for preservative effectiveness for their acceptable range.
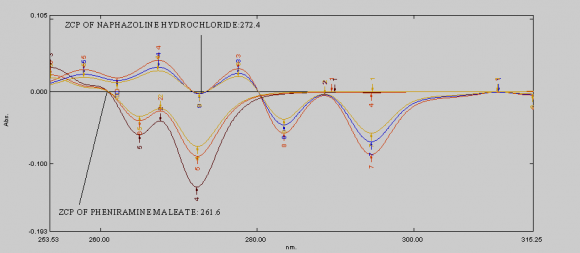
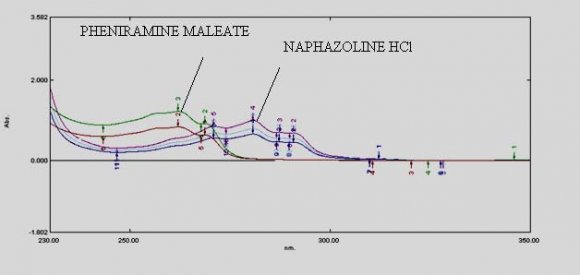
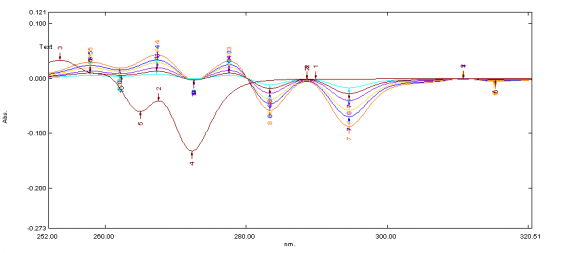
ix. Invitro Diffusion study The Franz diffusion cell was used for studying the in vitro release of the viscous eye care solution(drops). A cellulose acetate membrane (Dialysis membrane with 25 mm diameter) was adapted to the terminal portion of the cylindrical donor compartment. 2.5 mL viscous eye drops containing drug, sufficient for establishing sink conditions for the assay was placed into the donor compartment. The receptor compartment contained 15 mL of Phosphate buffer solution of pH 7.4 maintained at 37°C under mild agitation using a magnetic stirrer. At specific time intervals, aliquots of 3mL will be withdrawn and immediately restored with the same volume of fresh phosphate buffer. The amount of drug released was assessed by measuring the absorbance at 261.6 nm and 272.4nm for Naphazoline and Pheniramine respectively using UV spectrophotometers. In order to analyze the drug release mechanism, in vitro release data was fitted into a zero-order, first order, Higuchi, And Korsmeyerpeppas model.
x To assess the drug and formulation stability, stability studies was done according to ICH guidelines. Optimized formulation was kept in the stability chamber at specified temperature and humidity (40°±10°C and 75%RH) for one month. The chemical stability of the formulation was assessed by the estimation of the percentage drug remaining in the formulation and physical stability was evaluated by monitoring any change in pH, viscosity and appearance.
Volume XV Issue II Version I
7. Result and Discussion
Development of first order derivative spectroscopy method for simultaneous determination of NH and PM:-Selection of wavelength for simultaneous estimation of NH and PM :-
8. a) pH
The pH values for all formulations were shown in Table 4. The pH was within acceptable range (6.8 to 7.4) and would not cause any irritation upon administration of the formulation.
9. b) Refractive index
Refractive index of tear fluid is 1.340 to 1.360. (Hussein et al.2009) It is recommended that eye care solution should have refractive index values not higher than 1.476. Table 4 depicts that Naphazoline hydrochloride and Pheniramine maleate viscous eye care solution had refractive index values ranging from 1.443 to 1.334 which are within the recommended values. Data reflected that no visual problem may cause in patient after administration of formulation.
10. c) Viscosity
The longer contact time and effectiveness of the formulation in the eye is probably dependent not only on its mucoadhesive properties, but also on its viscosity and bulk rheological properties. Viscosity data of all the batches are shown in figures. Viscosity study in phosphate buffer 7.4 pH and in simulated tear fluid was carried out. From the rheograms shown in Fig. 14 & 15 medium remain same. From the data, it was found that batches F1, F4 and F7 shows very high viscosity due to highest concentration of NaCMC; was not selected as they were too viscous to instill properly into the eye. While, batches F6, F8 and F9 shows low viscosity due to lowest concentrations 0.5% HPMC, was not selected as aim of this study was to increase the viscosity of the formulations developed for residence time in ocular globe. Batches F3, F5 and F7 showed satisfactory viscosity. Each batch showed non-newtonian (dilatant) flow behaviour without any hysteresis. Non-newtonian solutions offer less resistance to movement of the eyelids over the globe and, therefore, are expected to be more comfortable in the eye than Newtonian solutions. By using the viscolysers like HPMC and NaCMC, viscosity of the prepared eye care solution was increased which gives longer contact time to the ocular globe. Therefore frequency of the instillation is decreased, which improves patient compliance. This increasing shear rate mimic ocular shear rates and respectively was found that viscosity in both the associated with normal blinking which is extremely wide, ranging from 0.03-28500S -1 . 7 and as per USP there should be 1 log reduction after 7 days, 3 log reductions after 14 days and no growth in population as compare to 14 th day after 28 days. In case of fungi, as per USP, there should be no growth/inhibition for AET. Optimized batch was obeyed the similar pattern of reduction in population as per standard limit and complies with the results.
11. Figure 14
12. f) Osmolarity
The Osmolarity of the ophthalmic preparation should be in the range of 310-350 mOsmol/kg to avoid irritation. Osmolarity of optimized batches was found to be in the range and shows 325 mOsmol/kg which was within the acceptable tonicity range of the eye to avoid ocular irritation. V.
13. g) Animal Study
14. Conclusion
Thus, on the basis of all the studies we can conclude that the batch having naphazoline hydrochloride and pheniramine maleate along with 0.59 % of HPMC E4M in the combination with 0.26 % NaCMC viscous solution may be considered as a promising for ophthalmic drug delivery.If the aforementioned formulation will scaled-up to manufacturing level, it will be used for potential sustained release effect for the allergic conjuctivitis.





| Independent Variables | Levels | ||
| Low | Medium | High | |
| X 1 = Concentration of HPMC E4M (% W/V) | 0.5 | 0.55 | 0.6 |
| X 2 = Concentration of NaCMC (% W/V) | 0.25 | 0.4 | 0.5 |
| Transformed values | -1 | 0 | 1 |
| Y 1 = Viscosity(cps) | |||
| Dependent variables | Y 2 = Mucoadhesive Index | ||
| Y 3 = Drug Release in 8 hr(%CDR) | |||
| a) Evaluation Parameters |
| i. Clarity |
| Clarity test was done by visual inspection of |
| each container and by measuring the refractive index |
| using refractometer at 25°c. |
| ii. PH |
| PH of prepared viscous eye care solution was |
| measured with pH meter. |
| iii. Viscosity |
| Viscosity of batches F1-F9 by Brookfield |
| viscometer at different RPM. By plotting graph of RPM |
| vs. viscosity, flow pattern was checked. |
| Name of components |
| Batch | Clarity | pH (n= 3) | Refractive Index |
| F1 | Slightly translucent | 7.40±0.02 | 1.469±0.001 |
| F2 | Clear | 7.40±0.01 | 1.431±0.001 |
| F3 | Clear | 7.41±0.01 | 1.356±0.001 |
| F4 | Slightly translucent | 7.40±0.01 | 1.465±0.002 |
| F5 | Clear | 7.40±0.03 | 1.358±0.002 |
| F6 | Clear | 7.40±0.01 | 1.341±0.001 |
| F7 | Slightly translucent | 7.41±0.01 | 1.471±0.001 |
| F8 | Clear | 7.41±0.02 | 1.341±0.002 |
| F9 | Clear | 7.40±0.01 | 1.334±0.001 |
| Batch | Media | Result | |
| ATGM | SBCD | ||
| F* | Clear | Clear | Complies |
| ATGM-Alternative Thio glycolate media; SBCD -Soya bean casein digest media | |||
| e) Antimicrobial efficacy testing (AET) | shown in Table | ||
| The microbial count for bacteria (Escherichia coli, | |||
| Pseudomonas aeruginosa, Staphylococcus aureus) is | |||
| Culture | Batch | Initial | 7 th day | 14 th day | 28 th day | |||||
| Candida albicans | F* | 15 x 10 | 6 | 85 x 10 | 5 | 46 x 10 | 4 | 33 x 10 | 3 | |
| Aspergillus Nniger | F* | 9 x 10 | 6 | 34 x 10 | 5 | 52 x 10 | ||||
| Culture | Batch | Initial | 7 th day | 14 th day | 28 th day | ||
| Escherichia Coli | F* | 25 x 10 | 5 | 18 x 10 | 4 | 300 | 253 |
| Pseudomonas aeruginosa | F* | 19 x 10 | 5 | 11 x 10 | 4 | 287 | 218 |
| Staphylococcus aureus | F* | 22 x 10 | 5 | 13 x 10 | 4 | 240 | 198 |
| FORMULATION | RABBIT A | RABBIT B | ||||||||||
| TIME (HR.) | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 |
| F* | N | N | N | N | N | N | - | - | - | - | - | - |
| CONTROL | N | N | N | N | N | N | - | - | - | - | - | - |
| OPCON A | - | - | - | - | - | - | N | N | N | N | N | N |
| CONTROL | - | - | - | - | - | - | N | N | N | N | N | N |
| Days | pH* | Assay of NH (% w/v)* | Assay of PM (% w/v)* | Assay of BKC (% w/v)* |
| 15 | 7.4 ± 0.02 | 99.60±0.54 | 99.34±0.34 | 99.79±0.64 |
| 30 | 7.41 ± 0.01 | 99.41±0.83 | 98.76±0.43 | 99.58±0.90 |
| *Value Expressed as Mean ± SD (n=3) | ||||