1. I. Introduction
erebrovascular accident (CVA) or stroke may result from multiple reasons including thrombus formation in the atherosclerotic cerebral blood vessels, hemorrhage due to rupture of a blood vessel (resulting from aneurysm), or due to a travelling clot which may block blood flow to a particular area in the brain [1]. CVA is one of the leading causes of mortality globally [2]. According to the World Health Organization (WHO), 15 million people suffer from stroke, of which 5 million die, and 5 million experience permanent disabilities as a result of stroke every year [3].
Several risk factors contribute to the occurrence of CVA. The Reasons for Geographic And Racial Differences in Stroke (REGARDS) study showed that Author ? ?: e-mails: [email protected], [email protected] smoking, poor diet, lack of physical activity, body mass total cholesterol, and high fasting blood glucose are the risk factors that may contribute to CVA [4]. In addition, the REGARDS study also showed that the cognitive skills of patients with stroke are compromised. Das et al demonstrated that the incidence of stroke related deaths is higher among the elderly [5].
There are multiple aspects of CVA treatment which begin from the time of first attack. The American Heart Association/American Stroke Association (AHA/ ASA) encourages education on stroke management to enhance early stroke detection and pre-hospital stroke management [6]. Effective neuroprotection can be achieved by initiating treatment of stroke within hours of injury [7]. Tissue plasminogen activator (tPA), anticoagulants, antiplatelet agents, vasodilators, neuroprotective agents, and surgical interventions are conventional therapeutic agents available for the management of stroke [8]. However; the use of these agents is considered helpful within few hours of stroke attack. Most patients with CVA continue to live a compromised life due to decreased quality of life (QoL), impaired cognitive skills, and several psychological symptoms [9,10].
Previous research has shown that stem cell therapy may help restore the neurological functions among patients with CVA. Bone marrow derived stem cells have demonstrated participation in neurogenesis and angiogenesis resulting in restoration of normal function [7]. Intracerebral transplantation of the neuronal stem cells in patients with stroke showed stable motor function even six months after transplantation [7]. Lindvall et al showed improved forelimb performance after transplantation of neuronal stem cells in stroke affected rodents [11]. Huang et al showed decrease in oxygen glucose deprivation and decrease in the rate of apoptosis via interlukin-6 and vascular endothelial growth factor signaling pathways after transplantation of mesenchymal stem cells (MSCs) [12].
Although several studies evaluating the effect of stem cells in the treatment of CVA are available, the evaluation of human embryonic stem cells (hESCs) is less explored. In the present study, we aimed to evaluate the efficacy and safety of hESC therapy on 22 patients with CVA.
2. II. Materials and Methods
3. a) Study Characteristics
The present single cohort study included patients with CVA and was conducted between 29 Dec 2004 and 03 Oct 2011 at a single site in New Delhi, India. This study evaluated the safety and efficacy of hESC therapy in patients with CVA.
The study protocol was approved by the Independent Institutional Ethics Committee. The institutional committee for stem cell research and therapy at our institute reported the clinical study to the National Apex Body and the Indian Council of Medical Research (ICMR). The study was conducted in accordance to the Declaration of Helsinki [7]. A written informed assent/consent was obtained from the patients/parents or legal guardians prior to the treatment.
4. b) Inclusion and Exclusion Criteria
Patients who approached our institute with a documented diagnosis of CVA and those who were willing to provide a written informed consent were included.
Patients who had previously received any other form of stem cell therapy simultaneously or less than a year of receiving hESC and patients who were not willing to provide a written informed consent were excluded. Pregnant and lactating women were also excluded.
5. c) Removal of Patients from Therapy
The patients who willingly wanted to discontinue the study were removed from therapy. Death of the patient or adverse events (AE) which were not related to hESC therapy led to discontinuation from the study. Cell Culture, Preparation, and Transplantation
The hESCs were derived from a primary cell line of pre-embryonic cell through two secondary cell lines derived by directed neuronal and non-neuronal differentiation of primary cell. The detailed cell culture technique has been described elsewhere (detailed compositions comprising human embryonic stem cells and their derivatives, methods of use, and methods of preparation is available at http://patentscope.wipo. int/search/en/WO2007141657). The cells have been characterized and are chromosomally stable [13].
6. Study Design
The study consisted of six treatment phases (T1, T2, T3, T4, T5, and T6), each phase separated by a gap phase. After the patients were diagnosed with CVA, the dosage and schedule of hESC was administered according to a protocol (Fig 1). The treatment schedule for each patient was individualized and modified as per the ongoing process of patient evaluation. Each patient was administered 0.05 mL hESCs subcutaneously to observe any hypersensitivity, pain or inflammation reactions at the site of injection for 24 hr. If the patient did not show any sign of hypersensitivity reactions 24 hr after the administration of test dose, the patient started to receive intensive dosing.
In T1, 0.25 mL hESCs were administered twice daily for 8 weeks. During T1, patients also received intravenous (IV) infusion of hESCs in 100 mL of normal saline which was repeated every 10 days and a priming dose of hESC by one of the supplemental routes by rotation (caudal injection, deep spinal injection, branchial plexus injection, and epidural route) for 5-14 days. hESCs were administered through the caudal route to ensure they reach the spinal fluid and regenerate the spinal cord and allow deep muscles to repair.
At the end of T1, the patients were discharged from the hospital and instructed to return for T2 and T3 which lasted for 4 weeks, each with a gap phase of 3 to 6 months in-between. During T2 and T3, the patients received 0.25 mL of hESC intramuscularly (IM), 1 mL of hESC every 10 days intravenously, and 1 dose of hESC every 7 days by supplemental routes by rotation as in T1. In addition to hESC therapy, all patients received physiotherapy and occupational therapy. The detailed treatment plan is illustrated in Figure 1.
7. d) Efficacy and Safety Evaluation
The efficacy variable included assessment of European Stroke Scale (ESS) in each patient at baseline and at the end of each treatment period [14]. This scoring system assessed the functional disability of the patients and the prognosis of the patients suffering from CVA.
8. Volume XV Issue II Version I
Year 2015
9. ( )
The ESS scores ranged from 0 (minimum score) to 100 (maximum score) and evaluated the patients on 14 parameters including consciousness, comprehension, speech, visual field, gaze, facial movement, arm in outstretched position, arm raising, extension of wrist, fingers, leg maintained in position, leg flexing, dorsiflexion of foot and gait. Each parameter of evaluation has different scores based on the extent to which the patient is affected. A completely normal patient would score 100 and a maximally affected person would score 0 on the ESS scale. In addition to ESS scores, we also analyzed improvement in other important parameters which are usually compromised in stroke patients scores developed in-house. These scores were used to assess the improvement in walking, balance (sitting and standing) and spasticity.
All AEs were documented during the study. In addition, the severity (1-mild; 2-moderate; 3-severe), seriousness, duration, nature of treatment or intervention to manage the event, and the outcome of the AE including the causality in the opinion of the investigator were documented.
10. e) Statistical Analysis
Intention to treat (ITT) population consisted of all patients who had received at least one test dose followed by intensive doses. The population included in the safety analysis was excluded from the efficacy evaluation. Only patients for whom all data was available were included in the statistical analysis. Patients with missing data were excluded. All demographic data of the patients was analyzed with descriptive statistics. The summary statistics of median, minimum and maximum values were presented. The AEs were summarized using number of patients (n), percentage (%) and system organ class (SOC) and preferred term (PT) for each study period and overall study period. A p value of less than 0.05 was considered to be statistically significant (5% level of significance). Statistical analysis was performed using software SPSS version 19 (IBM Corporation, Armonk, NY).
11. III. Results
12. a) Here Study Patients
A total of 22 patients were included in the study and all patients received intensive dosing with hESCs. Most of the patients included were males (63.6%) with a mean age of 61.8 yr. All the 22 patients received hESC during T1, 8 patients returned for T2, 6 patients returned for T3, 4 patients returned for T4, and only 2 patients each returned for T5 and T6. Of the 22 patients, 14 patients had received treatment only once, 2 patients each received 2, 3, 4, and 6 treatment phases.
13. Efficacy Evaluation b) ESS Scores
Median ESS scores increased from baseline through all the treatment periods indicating improvement in the condition of patients. The change in ESS scores at each treatment phase and the change from baseline are summarized in Table 1. At baseline, the median ESS score for the affected 22 patients was 61 (24,86). At the end of T1, the ESS score increased to 74 (42, 93). Eight patients returned for T2 and the ESS score at the end of this period was 67 (52, 92) for these patients. For T3, 6 patients returned and the ESS score was 67 (66, 79) at the end of this period. The ESS score at the end of T4 was 70 (66, 79) and 4 patients received hESC therapy in this period. A total of 2 patients each returned for T5 and T6 and the ESS scores at the end of these periods were 74 (72, 76) and 81 (76, 85), respectively. The change in the ESS score of each patient per treatment phase is presented in Table 2. All the 22 patients included in the present study had problem walking before receiving hESC therapy. However; after receiving hESC therapy an improvement in walking by at least one level occurred in a total of 21 patients. Of the 21 patients who had affected balance while standing, 20 patients showed an improvement by at least one level after receiving hESC therapy. In addition, all the affected patients showed improvement in balance while sitting (20 patients); and spasticity (17 patients).
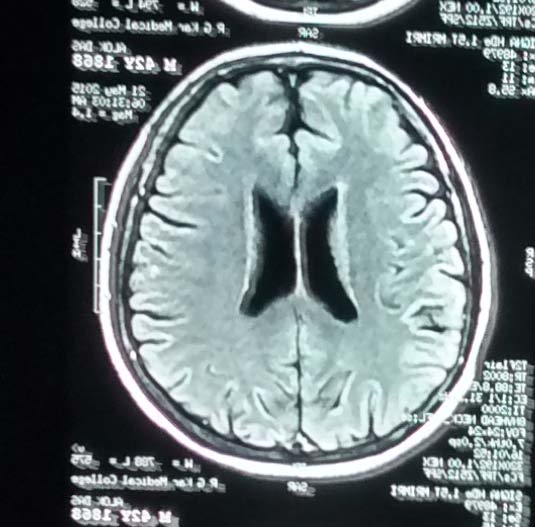
In addition to the ESS scores of the patients, all patients were evaluated for recovery using single photon emission computerized tomography (SPECT) scan and magnetic resonance imaging (MRI). At the end of therapy (T6) most patients showed improved perfusion on SPECT scan. (
14. c) Safety Evaluation
Overall, 4 patients experienced 11 AEs during the entire duration of the study. Of these, 3 patients experienced AEs in T1 and 1 patient experienced AEs in T2. However, no AEs were reported in T3, T4, T5 and T6. The most commonly experienced AEs included weakness/dizziness (3 patients, 27.3%); pain in shoulder, wrist, and joint (2 patients, 18.2%); constipation; fever; anxiety; blurred vision; diarrhea; and acidity (1 patient each, 9.1%). Of all the AEs experienced by the patients, 45% were mild and 55% were moderate in intensity. All AEs reported during the study period resolved within 48 hr. No serious adverse events (SAEs) and deaths were reported during the study (Table 4).
15. IV. Discussion
The present study has shown promising results among the patients with CVA after treatment with hESC therapy. Most of the patients included had difficulty in maintaining leg position, leg flexion, gait, arm outstretched position, raising of arms and fingers, foot dorsiflexion, wrist extension, and experienced difficulty in speech. However, these patients demonstrated an improvement in their condition after receiving hESC therapy. After receiving hESC therapy, an improvement by at least one level was noted in gait (22 patients); speech (15 patients); level of consciousness (2 patients); comprehension and gaze (1 patient each),. Improvement in these parameters showed a better QoL among most patients included in the present study. The patients who received hESC therapy at the early stages of CVA showed a better improvement in most aspects as compared with patients who received hESC therapy at later stages of CVA. SPECT scan done after the therapy showed normal perfusion as compared with SPECT scan done before the therapy. (Figure 2
16. and Fig 3).
Kondziolka et al evaluated the effect of neuronal stem cells on patients with stroke using ESS scores and found that mean total ESS scores of patients increased from 69.3 at baseline to 74.4 at 6 months. In addition, this study demonstrated an improvement in functional deficits among patients with stroke [15]. However, the results of our study showed an improvement in both functional and motor deficits using hESCs.
CVA is third among all the leading causes of death globally [8]. The increasing incidence of stroke has led to rising healthcare costs [16]. Although treatment of patients with stroke is available with tPA, it has a narrow time window (within 3 hr of onset) [17] and several contraindications due to which it is available to less than 5% stroke patients [18]. Decreased QoL makes it extremely difficult for these patients to perform their daily activities. According to a study conducted by Kim et al, patients with stroke have decreased functional independence, social interaction and reduced QoL [19]. Most of the patients with stroke are unable to walk and maintain balance while sitting and standing. According to the stroke association patients with stroke are unable to sit or stand while maintaining balance and may also experience trouble walking [20]. In a study conducted by Weerdt et al to identify different problems associated with stroke, 25.7% patients showed lack of active movement and general mobility and 19.5% patients showed imbalanced muscle tone [21] does not consider improvement in walking and balance. Therefore, we analyzed improvement in these parameters using an in-house scoring system. Most of the patients showed an improvement in walking, balance problems, and spasticity after receiving hESC therapy.
Stem cells possess self-renewal and multipotency features and have the ability to differentiate into any cell type in vivo. Of the different types of stem cells, embryonic stem cells are considered to possess the highest potential to give rise to any cell type and are referred to as pluripotent cells. Stem cells are capable of forming synaptic connections in the stroke injured brain after transplantation. Stem cells have neuroprotective properties which help reverse the damage caused by stroke [18].
Stem cell therapy is gaining more importance for the treatment of CVA. Most studies conducted to evaluate the effect of stem cell therapy suggest that stem cells have a neuroprotective effect. Chen et al demonstrated that adipose tissue-derived stem cells (ADSCs) restore brain function through several mechanisms including secretion of vascular endothelial growth factor (VEGF) for angiogenesis of the injured region, stimulation of brain repair markers and reduction of brain injury derived apoptosis [22].
A study conducted by Chang et al showed that hESC derived neural precursor cells (NPCs) migrate and survive in the infarct region and show improvement in functional deficits in rodents with ischemic stroke. This study reported that improved functional deficits may be associated with neurorestorative and neuroprotective effects of the transplanted hESC-NPCs [23]. Another study showed that hESC derived neural progenitor cells improved regenerative activities and sensory function without immune suppression when transplanted into the ischemic core and penumbra region after ischemic stroke in an animal model [24].
There are very few studies which have shown the effect of hESCs in the treatment of stroke. The difficulty in isolation of hESCs has restricted evaluation of their potential in the treatment of CVA. After being transplanted, hESCs grow in the affected area to replace the degenerated cell type. hESCs regenerate damaged cells by communicating with the damaged area and "homing" in the site of injury. This often occurs by the release of chemokines, cytokines, and growth factors from the site of injury [25]. In addition, the route of administration was selected as this shows an impact on the migration of hESCs and their ability to "home" in the damaged tissue. MSCs have also shown the influence of administration route on their potential to migrate and home at the site of injury [26]. The route of administration and the dosing schedule of hESCs play a vital role in their mechanism of action. The IM and IV routes were used to facilitate faster migration and transplantation of the hESCs to the affected area. A gap phase was included between each treatment period to facilitate the process of homing, and regeneration within the body. Adequate time intervals were maintained between each treatment period for the elucidation of maximum effect in the affected areas of the brain. Patients were treated in subsequent treatment phases after carefully monitoring the extent of improvement with MRI and SPECT scan. In the present study, we used hESCs which were isolated using a patented in-house technique for the treatment of patients (United States Granted Patent-WO 2007/141657A PCT/1B 2007 Published 13 Dec 2007). These cells do not have any xeno-product in them.
V.
17. Conclusion
In conclusion, the results of the present study have demonstrated effective improvement in the condition of patients with CVA. Most patients showed improved cognitive skills and regained their functional ability which improved the QoL of the patients included in the study. Although AEs were reported, no patients experienced severe AEs or SAEs during the study as a result of hESC therapy. hESC therapy was well tolerated among all the patients included in the study. However; further large scale prospective studies are required for making hESC therapy available clinically for patients with CVA.
18. Volume XV Issue II Version I

| Treatment Period | N | End Period Score Median | Change from Baseline |
| (Min, Max) | Median (Min, Max) | ||
| Baseline | 22 | 61 (24, 96) | - |
| T1 | 22 | 74 (42, 93) | 14 (0, 20) |
| T2 | 8 | 67 (52, 92) | 22 (3, 38) |
| T3 | 6 | 67 (66, 79) | 24 (3, 42) |
| T4 | 4 | 70 (66, 79) | 22 (3, 42) |
| T5 | 2 | 74 (72, 76) | 14 (8, 19) |
| T6 | 2 | 81 (76, 82) | 20 (19, 21) |
| Global Journal of Medical Research | ||||||||
| Sl.No | Age | Baseline | T1 | T2 | T3 | T4 | T5 | T6 |
| 1 | 25 | 64 | 78 | - | - | - | - | - |
| 2 | 32 | 64 | 64 | 67 | 67 | 67 | 72 | 85 |
| 3 | 41 | 73 | 79 | - | - | - | - | - |
| 4 | 42 | 52 | 64 | 67 | 71 | 79 | - | - |
| 5 | 56 | 66 | 77 | - | - | - | - | - |
| 6 | 56 | 50 | 64 | 66 | 79 | - | - | - |
| 7 | 57 | 52 | 72 | - | - | - | - | - |
| Parameter | Number of Patients | Number of Patients Showing Improvement |
| Affected at Baseline | by at least 1 level n (%) | |
| Leg maintain position | 22 | 19 (86.4) |
| Leg flexion | 22 | 20 (90.9) |
| Gait | 22 | 22 (100) |
| Arm outstretched position | 21 | 19 (86.4) |
| Arm raising | 21 | 20 (95.2) |
| Fingers | 21 | 12 (57.1) |
| Foot Dorsiflexion | 21 | 16 (76.2) |
| Wrist Extension | 20 | 14 (70) |
| Speech | 15 | 15 (100) |
| Facial movements | 11 | 10 (90.9) |
| Level of consciousness | 2 | 2 (100) |
| Visual field | 2 | 1 (50) |
| Comprehension | 1 | 1 (100) |
| Gaze | 1 | 1 (100) |
| Adverse Event | Number of Events (%) |
| Weakness/dizziness | 3 (27.3) |
| Pain (shoulder, wrist, and joint) | 2 (18.2) |
| Constipation | 1 (9.1) |
| Fever | 1 (9.1) |
| Anxiety | 1 (9.1) |
| Blurred Vision | 1 (9.1) |
| Diarrhea | 1 (9.1) |
| Acidity | 1 (9.1) |